Abstract
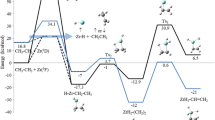
The two-state mechanism of the reaction of Nb(NH2)3 with N2O on the singlet and triplet potential energy surfaces has been investigated at the B3LYP level. Crossing points between the potential energy surfaces have been located using different methods. Analysis of the strain model shows that the singlet state of the four-coordinate (N2O)Nb(NH2)3 complex with N2O bonded via terminal N atom coordination (1 2) is more stable in the initial stage of reaction, since the bending of the N2O fragment [E def(N2O) = 86.1 kcal mol−1] results in an energy splitting of the doubly degenerate LUMO; the low-energy LUMO can now strongly couple with the occupied Nb-localized d orbitals, forming a back-bond and transferring charge (q = 0.82 e) from Nb(NH2)3 to the N2O ligand. Going from 3 2 to 1 2, the reacting system changes spin multiplicity near the MECP (minimal energy crossing point) region, which takes place with a spin crossing barrier of 9.6–10.0 kcal mol−1. Analysis of spin-orbit coupling (SOC) indicates that MECP will produce a significant SOC matrix element. The value of SOC is 111.52 cm−1, due to the electron shift between two perpendicular ϕ orbitals with the same rotation direction, and the magnitude of the spin-multi-plicity mixing increases in the small energy gap between high- and low-spin states, greatly enhancing the probability of intersystem crossing. The probabilities of single (P ISC1 ) and double (P ISC2 ) passes estimated at MECP (SOC = 111.52 cm−1) are approximately 1.17×10−2 and 2.32×10−2, respectively.
Similar content being viewed by others
References
Prather RA, Ehhalt DH. In: Houghton, JT, Joos F, McFarlan M. Eds. Climate Change 2001: The Scientific Basis. New York: Cambridge University Press, 2001
Khoroshun DV, Musaev DG, Morokuma K. Does reaction of three-coordinate molybdenum(III) with N2O proceed via the same mechanism as with N2? A theoretical study. Organometallics, 1999, 18: 5653–5660
Mindiola DJ, Meyer K, Cherry JPF, Baker TA, Cummins CC. Dinitrogen cleavage stemming from a heterodinuclear niobium/molybdenum N2 complex: New nitridoniobium systems including a niobazene cyclic trimer. Organometallics, 2000, 19: 1622–1624
Graham DC, Beran GJO, Head-Gordon M, Christian G, Stranger R, Yates BF. Nitrogen activation via three-coordinate molybdenum complexes: Comparison of density functional theory performance with wave function based methods. J Phys Chem A, 2005, 109: 6762–6772
Johnson AR, Davis WM, Cummins CC, Serron S, Nolan SP, Musaev DG, Morokuma K. Four-coordinate molybdenum chalcogenide complexes relevant to nitrous oxide N-N bond cleavage by three-coordinate molybdenum(III): Synthesis, characterization, reactivity, and thermochemistry. J Am Chem Soc, 1998, 120: 2071–2085
Greco JB, Peter JC, Baker TA, Davis WM, Cummins CC, Wu G. Atomic carbon as a terminal ligand: Studies of a carbidomolybdenum anion featuring solid-state 13C NMR data and proton-transfer self-exchange kinetics. J Am Chem Soc, 2001, 123: 5003–5013
Peters JC, Baraldo LM, Baker TA, Johnson AR, Cummins CC. Dimolybdenum-μ-cyanide complexes supported by N-tert-butylanilide ligation: In pursuit of cyanide reductive cleavage. J Organomet Chem, 1999, 591: 24–35
Ariafard A, Brookes NJ, Stranger R, Yates BF. Activation of CS2 and CS by ML3 complexes. J Am Chem Soc, 2008, 130: 11928–11938
Fickes MG, Odom AL, Cummins CC. A nucleophilic niobium(V) nitride prepared by isocyanate decarbonylation. Chem Commun, 1997, 1993–1994
Cherry JPF, Johnson AR, Baraldo LM, Tsai YC, Cummins CC, Kryatov SV, Rybak-Akimova EV, Capps KB, Hoff CD, Haar CM, Nolan SP. On the origin of selective nitrous oxide N-N bond cleavage by three-coordinate molybdenum(III) complexes. J Am Soc Chem, 2001, 123: 7271–7286
Christian GJ, Stranger R, Yates BF. Optimizing small molecule activation and cleavage in three-coordinate M[N(R)Ar]3 complexes. Inorg Chem, 2006, 45: 6851–6859
Heinemann C, Cornehl HH, Schroder D, Dolg M, Schwarz H. The CeO +2 cation: Gas-phase reactivity and electronic structure. Inorg Chem, 1996, 35: 2463–2475
Fiedler A, Schroder D, Shaik S, Schwarz H. Electronic structures and gas phase reactivities of cationic late-transition-metal oxides. J Am Chem Soc, 1994, 116: 10734–10741
Yoshizawa K, Shiota Y, Yamabe T. Intrinsic reaction coordinate analysis of the conversion of methane to methanol by an iron-oxo species: A study of crossing seams of potential energy surfaces. J Chem Phys, 1999, 111: 538–545
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Jr Montgomery JA, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Menncci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Malick D. K, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Stefanov BB, Liu G., Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Gonzalelez C, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andres JL, Gonzalez C, Head-Gordon M, Replogle ES, Pople JA. Gaussian 03 (Revision-E.01), Gaussian Inc., Pittsburgh PA, 2003
Harvey JN, Aschi M, Schwarz H, Koch W. The singlet and triplet states of phenyl cation. A hybrid approach for locating minimum energy crossing points between non-interacting potential energy surfaces. Theor Chem Acc, 1998, 99: 95–99
The version for personal computers was compiled by Granovsky AA, Moscow State University
Danovich D, Marian CM, Neuheuser T, Peyerimhoff SD, Shaik S. Spin-orbit coupling patterns induced by twist and pyramidalization modes in C2H4: A quantitative study and a qualitative analysis. J Phys Chem A, 1998, 102: 5923–5936
Isobe H, Yamanaka S, Kuramitsu S, Yamaguchi K. Regulation mechanism of spin-orbit coupling in charge-transfer-induced luminescence of imidazopyrazinone derivatives. J Am Chem Soc, 2008, 130: 132–149
Koseki S, Fedorov DG, Schmidt MW, Gordon MS. Spin-orbit splittings in the third-row transition elements: Comparison of effective nuclear charge and full Breit-Pauli calculations. J Phys Chem A, 2001, 105: 8262–8268
Lower SK, El-Sayed MA. The triplet state and molecular electronic processes in organic molecules. Chem Rev, 1966, 66: 199–241
Gorelsky S, Ghosh S, Solomon EI. Mechanism of N2O reduction by the u 4-S tetranuclear cluster of nitrous oxide reductase. J Am Chem Soc, 2006, 128: 278–290
Tuan DFT, Hoffmann R. Nitrogen atom vs. oxygen atom linkage and σ- vs. π-bonding in transition-metal complexes of dinitrogen oxide and cyanate. Inorg Chem, 1985, 24: 871–876
Hirao H, Kumar D, Que Jr L, Shaik S. Two-state reactivity in alkane hydroxylation by non-heme iron-oxo complexes. J Am Chem Soc, 2006, 128: 8590–8606
Matsushita T, Asada T, Koseki S. Relativistic study on emission mechanism in palladium and platinum complex. J Phys Chem A, 2006, 110: 13295–13302
Zener C. Non-adiabatic crossing of energy levels. Proc R Soc London Ser A, 1932, 137: 696–702
Zener C. Dissociation of excited diatomic molecules by external perturbations. Proc R Soc London Ser A, 1933, 140: 660–668
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lv, L., Wang, X., Zhu, Y. et al. Theoretical study of spin-orbit coupling and intersystem crossing in the two-state reaction between Nb(NH2)3 and N2O. Sci. China Chem. 55, 158–166 (2012). https://doi.org/10.1007/s11426-011-4468-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-011-4468-7