Abstract
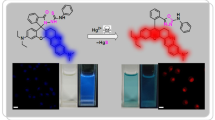
Borondipyrromethenes (BODIPY) are a class of fluorescent dyes whose fluorescence quantum yields are generally high and independent of the solvent. In this paper, we report the synthesis of a new type of BODIPY compound that carries an azido group on the 3-position of the pyrrole core. The azido group quenches the fluorescence of the dye due to its weak electron-donating effect. The fluorescence of the BODIPY dye can be switched on after reacting with alkynes via a Cu(I) catalyzed azide-alkyne cycloaddition (CuAAC) reaction. We further demonstrate that this azido-BODIPY compound can be used in the cell imaging applications.
Similar content being viewed by others
References
Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew Chem Int Ed, 2002, 41: 2596–2599
Tornoe CW, Christensen C, Meldal M. Peptidotriazoles on solid phase: [1xxx2xxx3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J Org Chem, 2002, 67: 3057–3064
Sivakumar K, Xie F, Cash BM, Long S, Barnhill HN, Wang Q. A fluorogenic 1,3-dipolar cycloaddition reaction of 3-azidocoumarins and acetylenes. Org Lett, 2004, 6: 4603–4606
Zhou Z, Fahrni CJ. A fluorogenic probe for the copper(I)-catalyzed azide-alkyne ligation reaction: Modulation of the fluorescence emission via (3)(n,pi*)-(1)(pi,pi*) inversion. J Am Chem Soc, 2004, 126: 8862–8863
Sawa M, Hsu TL, Itoh T, Sugiyama M, Hanson SR, Vogt PK, Wong CH. Glycoproteomic probes for fluorescent imaging of fucosylated glycans in vivo. Proc Nat Acad Sci USA, 2006, 103: 12371–12376
Xie F, Sivakumar K, Zeng QB, Bruckman MA, Hodges B, Wang Q. A fluorogenic “click” reaction of azidoanthracene derivatives. Tetrahedron, 2008, 64: 2906–2914
Beatty KE, Liu JC, Xie F, Dieterich DC, Schuman EM, Wang Q, Tirrell DA. Fluorescence visualization of newly synthesized proteins in mammalian cells. Angew Chem Int Ed, 2006, 45: 7364–7367
Le Droumaguet C, Wang C, Wang Q. Fluorogenic click reaction. Chem Soc Rev, 2010, 39: 1233–1239
Bergstrom F, Mikhalyov I, Hagglof P, Wortmann R, Ny T, Johansson LBA. Dimers of dipyrrometheneboron difluoride (BODIPY) with light spectroscopic applications in chemistry and biology. J Am Chem Soc, 2002, 124: 196–204
Trieflinger C, Rurack K, Daub M. “Turn ON/OFF your LOV light”: Boron-dipyrromethene-flavin dyads as biomimetic switches derived from the LOV domain. Angew Chem Int Ed, 2005, 44: 2288–2291
Zhao WL, Carreira EM. Conformationally restricted aza-bodipy: A highly fluorescent, stable, near-infrared-absorbing dye. Angew Chem Int Ed, 2005, 44: 1677–1679
Harriman A, Izzet G, Ziessel R. Rapid energy transfer in cascade-type bodipy dyes. J Am Chem Soc, 2006, 128: 10868–10875
Sapsford KE, Berti L, Medintz IL. Materials for fluorescence resonance energy transfer analysis: Beyond traditional donor-acceptor combinations. Angew Chem Int Ed, 2006, 45: 4562–4588
Liras M, Prieto JB, Pintado-Sierra M, Arbeloa FL, Garcia-Moreno I, Costela A, Infantes L, Sastre R, Amat-Guerri F. Synthesis, photophysical properties, and laser behavior of 3-amino and 3-acetamido BODIPY dyes. Org Lett, 2007, 9: 4183–4186
Riddle JA, Jiang X, Huffman J, Lee D. Signal-amplifying resonance energy transfer: A dynamic multichromophore array for allosteric switching. Angew Chem Int Ed, 2007, 46: 7019–7022
Nagai A, Miyake J, Kokado K, Nagata Y, Chujo Y. Highly luminescent BODIPY-based organoboron polymer exhibiting supramolecular self-assemble structure. J Am Chem Soc, 2008, 130: 15276–15278
Kaloudi-Chantzea A, Karakostas N, Raptopoulou CP, Psycharis V, Saridakis E, Griebel J, Hermann R, Pistolis G. Coordination-driven self assembly of a brilliantly fluorescent rhomboid cavitand composed of bodipy-dye subunits. J Am Chem Soc, 2010, 132: 16327–16329
Nepomnyashchii AB, Cho S, Rossky PJ, Bard AJ. Dependence of electrochemical and electrogenerated chemiluminescence properties on the structure of bodipy dyes unusually large separation between sequential electron transfers. J Am Chem Soc, 2010, 132: 17550–17559
Nepomnyashchii AB, Broring M, Ahrens J, Bard AJ. Synthesis, photophysical, electrochemical, and electrogenerated chemiluminescence studiesMultiple sequential electron transfers in BODIPY monomers, dimers, trimers, and polymer. J Am Chem Soc, 2011, 133: 8633–45
Whited MT, Djurovich PI, Roberts ST, Durrell AC, Schlenker CW, Bradforth SE, Thompson ME. Singlet and triplet excitation management in a bichromophoric near-infrared-phosphorescent BODIPY-benzoporphyrin platinum complex. J Am Chem Soc, 2011, 133: 88–96
Yogo T, Urano Y, Ishitsuka Y, Maniwa F, Nagano T. Highly efficient and photostable photosensitizer based on BODIPY chromophore. J Am Chem Soc, 2005, 127: 12162–12163
Hagihara S, Miyazaki A, Matsuo I, Tatami A, Suzuki T, Ito Y. Fluorescently labeled inhibitor for profiling cytoplasmic peptide:N-glycanase. Glycobiology, 2007, 17: 1070–1076
Bricks JL, Kovalchuk A, Trieflinger C, Nofz M, Buschel M, Tolmachev AI, Daub J, Rurack K. On the development of sensor molecules that display Fe-III-amplified fluorescence. J Am Chem Soc, 2005, 127: 13522–13529
Qin WW, Baruah M, Stefan A, Van der Ameraer M, Boens N. Photophysical properties of BODIPY-derived hydroxyaryl fluorescent pH probes in solution. Chem Physchem, 2005, 6: 2343–2351
Peng XJ, Du JJ, Fan JL, Wang JY, Wu YK, Zhao JZ, Sun SG, Xu T. A selective fluorescent sensor for imaging Cd2+ in living cells. J Am Chem Soc, 2007, 129: 1500–1501
Lee JS, Kang NY, Kim YK, Samanta A, Feng SH, Kim HK, Vendrell M, Park JH, Chang YT. Synthesis of a BODIPY library and its application to the development of live cell glucagon imaging probe. J Am Chem Soc, 2009, 131: 10077–10082
Devaraj NK, Hilderbrand S, Upadhyay R, Mazitschek R, Weissleder R. Bioorthogonal turn-on probes for imaging small molecules inside living cells. Angew Chem Int Ed, 2010, 49: 2869–2872
Nierth A, Kobitski AY, Nienhaus GU, Jaschke A. Anthracene-BODIPY dyads as fluorescent sensors for biocatalytic diels-alder reactions. J Am Chem Soc, 2010, 132: 2646–2654
Ulrich G, Ziessel R, Harriman A. The chemistry of fluorescent bodipy dyes: Versatility unsurpassed. Angew Chem Int Ed, 2008, 47: 1184–1201
Goze C, Ulrich G, Charbonnière L, Cesario M, Prangé T, Ziessel R. Cation sensors based on terpyridine-functionalized boradiazaindacene. Chem Eur J, 2003, 9: 3748–3755
Qin WW, Rohand T, Baruah M, Stefan A, Van der Auweraer M, Dehaen W, Boens N. Solvent-dependent photophysical properties of borondipyrromethene dyes in solution. Chem Phys Lett, 2006, 420: 562–568
Rohand T, Baruah M, Qin WW, Boens N, Dehaen W. Functionalisation of fluorescent BODIPY dyes by nucleophilic substitution. Chem Commun, 2006, 266–268
Rohand T, Qin WW, Boens N, Dehaen W. Palladium-catalyzed coupling reactions for the functionalization of BODIPY dyes with fluorescence spanning the visible spectrum. Eur J Org Chem, 2006, 4658–4663
Rurack K, Kollmannsberger M, Daub J. Molecular switching in the near infrared (NIR) with a functionalized boron-Dipyrromethene dye. Angew Chem Int Ed, 2001, 40: 385–387
Basaric N, Baruah M, Qin WW, Metten B, Smet M, Dehaen W, Boens N. Synthesis and spectroscopic characterisation of BODIPY (R) based fluorescent off-on indicators with low affinity for calcium. Org Biomol Chem, 2005, 3: 2755–2761
Qi X, Jun EJ, Xu L, Kim SJ, Hong JSJ, Yoon YJ, Yoon JY. New BODIPY derivatives as OFF-ON fluorescent chemosensor and fluorescent chemodosimeter for Cu2+: Cooperative selectivity enhancement toward Cu2+. J Org Chem, 2006, 71: 2881–2884
Zeng L, Miller EW, Pralle A, Isacoff EY, Chang CJ. A selective turn-on fluorescent sensor for imaging copper in living cells. J Am Chem Soc, 2006, 128: 10–11
Miller EW, Albers AE, Pralle A, Isacoff EY, Chang CJ. Boronate-based fluorescent probes for imaging cellular hydrogen peroxide. J Am Chem Soc, 2005, 127: 16652–16659
Yuan MJ, Li YL, Li JB, Li CH, Liu XF, Lv J, Xu JL, Liu HB, Wang S, Zhu D. A colorimetric and fluorometric dual-modal assay for mercury ion by a molecule. Org Lett, 2007, 9: 2313–2316
Bozdemir OA, Guliyev R, Buyukcakir O, Selcuk S, Kolemen S, Gulseren G, Nalbantoglu T, Boyaci H, Akkaya EU. Selective manipulation of ict and pet processes in styryl-bodipy derivatives: applications in molecular logic and fluorescence sensing of metal ions. J Am Chem Soc, 2010, 132: 8029–8036
Littler BJ, Miller MA, Hung CH, Wagner RW, O'shea DF, Boyle PD, Lindsey JS. Refined synthesis of 5-substituted dipyrromethanes. J Org Chem, 1999, 64: 1391–1396
Cornforth JW, Firth ME. Identification of two chromogens in the Elson-Morgan determination of hexosamines. A new synthesis of 3-methylpyrrole. Structure of the “pyrrolene-phthalides”. J Chem Soc, 1958, 1091–1099
Baruah M, Qin WW, Basaric N, De Borggraeve WM, Boens N. BODIPY-based hydroxyaryl derivatives as fluorescent pH probes. J Org Chem, 2005, 70: 4152–4157
De P, Gondi SR, Sumerlin BS. Folate-conjugated thermoresponsive block copolymers: Highly efficient conjugation and solution self-assembly. Biomacromolecules, 2008, 9: 1064–1070
Saeed AO, Magnusson JP, Moradi E, Soliman M, Wang W, Stolnik S, Thurecht KJ, Howdle SM, Alexander C. Modular construction of multifunctional bioresponsive cell-targeted nanoparticles for gene delivery. Bioconjugate Chem, 2011, 22: 156–168
Leamon CP, Reddy JA. Folate-targeted chemotherapy. Adv Drug Delivery Rev, 2004, 56: 1127–1141
Suthiwangcharoen N, Li T, Li K, Thompson P, You S, Wang Q. M13 bacteriophage-polymer nanoassemblies as drug delivery vehicles. Nano Res, 2011, 1–11
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, C., Xie, F., Suthiwangcharoen, N. et al. Tuning the optical properties of BODIPY dye through Cu(I) catalyzed azide-alkyne cycloaddition (CuAAC) reaction. Sci. China Chem. 55, 125–130 (2012). https://doi.org/10.1007/s11426-011-4452-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-011-4452-2