Abstract
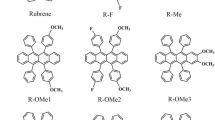
A novel pseudo rubrene analogue, 6,11-di(thiophen-2-yl)-tetracene-5,12-dione (DTTDO) was synthesized, in which two thienyl groups and two carbonyl groups replacing four phenyl groups in the rubrene molecule were connected to the backbone of tetracene. This compound was characterized by single crystal X-ray structure analysis, thermogravimetric analysis, absorption spectra and electrochemical measurements. Unlike rubrene, DTTDO exhibited excellent film forming ability by normal vacuum deposition, indicating its promising applications in organic thin film transistors.
Similar content being viewed by others
References
Klauk H. Organic thin-film transistors. Chem Soc Rev, 2010, 39: 2643
Dong HL, Wang CL, Hu WP. High performance organic semiconductors for field-effect transistors. Chem Commun, 2010, 46: 5211–5222
Jiang L, Dong HL, Hu WP. Organic single crystal field-effect transistors: Advances and perspectives. J Mater Chem, 2010, 20: 4994–5007
Seo S, Park BN, Evans PG. Ambipolar rubrene thin-film transistors. Appl Phys Lett, 2006, 88: 232114
de Boer RWI, Gershenson ME, Morpurgo AF, Podzorov V. Organic single-crystal field-effect transistors. Phys Status Solid A, 2004, 201: 1302
da Silva DA, Kim EG, Bredas JL. Transport properties in the rubrene crystal: Electronic coupling and vibrational reorganization energy. Adv Mater, 2005, 17: 1072–1076
Sundar VC, Zaumseil J, Podzorov V, Menard E, Willett R L, Someya T, Gershenson ME, Rogers JA. Elastomeric transistor stamps: Reversible probing of charge transport in organic crystals. Science, 2004, 303: 1644–1646
Takeya J, Yamagishi M, Tominari Y, Hirahara R, Nakazawa Y, Nishikawa T, Kawase T, Shimoda T, Ogawa S. Very high-mobility organic single-crystal transistors with in-crystal conduction channels. Appl Phys Lett, 2007, 90: 102120
Hsu CH, Deng J, Staddon CR, Beton PH. Growth front nucleation of rubrene thin films for high mobility organic transistors. Appl Phys Lett, 2007, 91: 193505
Park SW, Jeong SH, Choi JM, Hwang JM, Kim JH, Ima S. Rubrene polycrystalline transistor channel achived through in situ vacuum annealing. Appl Phys Lett, 2007, 91: 033506
Chena Y, Shih I. High mobility organic thin films transistors based on monocrystalline rubrene films grown by low pressure hot wall deposition. Appl Phys Lett, 2009, 94: 083304
Campione M. Rubrene heteroepitaxial nanostructures with unique orientation. J Phys Chem C, 2008, 112, 16178-16181
Seo JH, Park DS, Cho SW, Kim CY, Jang WC, Whang CN, Yoo KH, Chang GS, Pedersen T, Moewes A, Chae KH, Cho SJ. Buffer layer effect on the structural and electrical properties of rubrene-based organic thin-film transistors. Appl Phys Lett, 2006, 89: 163505
Choi JM, Jeong SH, Hwang DK, Im S, Lee BH, Sung MM. Rubrene thin-film transistors with crystalline channels achievedon optimally modified dielectric surface. Org Electron, 2009, 10: 199–204
Li Z, Du J, Tang Q, Wang F, Xu JB, Yu JC, Miao Q. Induced crystallization of rubrene in thin-film transistors. Adv Mater, 2010, 22: 3242–3246
Zeng XH, Wang LD, Duan L, Qiu Y. Homoepitaxy growth of wellordered rubrene thin films. Cryst Growth Des, 2008, 8: 1617–1622
Stingelin-Stutzmann N, Smits E, Wondergem H, Tanase C, Blom P, Smith P, De Leeuw D. Organic thin-film electronics fromvitreous solution-processed rubrene hypereutectics. Nat Mater, 2005, 4: 601–606
Chi XL, Li DW, Zhang HQ, Chen YS, Garcia V, Garcia C, Siegrist T. 5,6,11,12-Tetrachlorotetracene, a tetracene derivative with pi-stacking structure: The synthesis, crystal structure and transistor properties. Org Electron, 2008, 9: 234–240
Hou YH, Chi XL, Wan XJ, Chen YS. Synthesis and crystal structure of 5,12-diphenyl-6,11-bis(thien-2-yl)tetracene. J Mol Struct, 2008, 889: 265–270
Hauser C R, Tetenbaum MT, Hoffenberg DS. Condensations involving the metalation of the 3-position of 3-phenylphthalide by means of alkali amides — carbonation of phthalide. J Org Chem, 1958, 23: 861–865
Mohanakrishnan AK, Lakshmikantham MV, McDougal CM, Cava P, Baldwin JW, Metzger RM. Studies in the dithienylbenzo[c]thiophene series. J Org Chem, 1998, 63: 3105–3112
Amaladass P, Clement JA, Mohanakrishnan AK. Synthesis and characterization of benzannelated thienyl oligomers. Eur J Org Chem, 2008: 3798-3810
Mohanakrishnan AK, Amaladass P. Synthesis of 1,3-diaryl benzo [c]thiophenes. Tetrahedron Lett, 2005, 46: 4225–4229
Amaladass P, Kumar NS, Mohanakrishnan AK. Synthesis and characterization of 1,3-diarylbenzo[c]selenophenes. Tetrahedon, 2008, 64: 7992–7998
Song J, Wang J, Xu N. Preparation of a new derivate of rubrene. China patent, 200710037118.1, 2007-02-02
Dodge JA, Bain J, Chamberlin AR. Regioselective synthesis of substituted rubrenes. J Org Chem, 1990, 55: 4190–4198
Irwin C, Lewis ET. Thermal reactivity of polynuclear aromatic hydrocarbons. J Org Chem, 1963, 28: 2050–2057
Keszthelyi, Csaba P, Bard. Allen J. Electrogenerated chemiluminescence. XIX. Preparation and chemiluminescence of 5,12-dibromo-5,12-dihydro-5,6,11,12-tetraphenylnaphthacenel. J Org Chem, 1974, 39: 2936–2937
Hsu CH, Deng J, Staddon CR, Beton PH. Growth front nucleation of rubrene thin films for high mobility organic transistors. Appl Phys Lett, 2007, 91: 193505
Park B, In I, Gopalan P, Evans PG, King S, Lyman PF. Enhanced hole mobility in ambipolar rubrene thin film transistors on polystyrene. Appl Phys Lett, 2009, 92: 133302
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, X., Meng, Q., He, Y. et al. A new pseudo rubrene analogue with excellent film forming ability. Sci. China Chem. 54, 631–635 (2011). https://doi.org/10.1007/s11426-011-4234-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-011-4234-x