Abstract
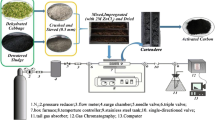
The low-cost activated carbon was prepared from a renewable aquatic plant residue, Trapa natans husk, and tested for its ability to remove norfloxacin (NOR) from aqueous solutions. Physical and chemical properties of the Trapa natans husk activated carbon (TAC) were characterized. TAC has a large surface area of 1274 m2/g and mesoporous structure. Carboxylic and hydroxyl groups contributed to the sorption of NOR onto TAC but they were not the most important factors in the sorption process. The rates of adsorption followed the pseudo-second-order kinetics and the overall rate of NOR uptake was controlled by both external mass transfer and intro particle diffusion during the entire adsorption period. The equilibrium data fitted well with the Freundlich and Tempkin models and the sorption was found to be a favorable process. The adsorption of NOR by TAC was strongly dependent on the solution pH. Electrostatic interaction and hydrophobic interaction were proposed to be the principal NOR sorption mechanism.
Similar content being viewed by others
References
Nmmerer KK. Pharmaceuticals in the Environment: Sources, Fate, Effects and Risks. 2nd ed. Berlin: Springer-Verlag, 2004
Halling-Scrensen B, Nielsen SN, Lanzky PF, Ingerslev F, Holten Lutzhoft HC, Jorgensen SE. Occurrence, fate and effects of pharmaceutical substances in the environment—A review. Chemosphere, 1998, 36: 357–393
Hirsch R, Ternes TA, Haberer K, Kratz KL. Occurrence of antibiotics in the environment. Sci Tot Environ, 1999, 225: 109–118
Golet EM, Xifra I, Siegrist H, Alder AC, Giger W. Environmental exposure assessment of fluoroquinolone antibacterial agents fromsewage to soil. Environ Sci Technol, 2003, 37: 3243–3249
Zhang J, Dong Y. Effect of low-molecular-weight organic acids on the adsorption of norfloxacin in typical variable charge soils of China. J Hazard Mater, 2008, 151: 833–839
Paul T, Miller PL, Strathman TJ. Visible-light-mediated TiO2 photocatalysis of fluoroquinolone antibacterial agents. Environ Sci Technol, 2007, 41: 4720–4727
Dodd MC, Shan AD, Gunten HAH U, Huang C. Interactions of fluoroquinolone antibacterial agents with aqueous chlorine: Reaction kinetics, mechanisms, and transformation pathways. Sci Technol, 2005, 39: 7065–7076
Zhang H, Huang C. Adsorption and oxidation of fluoroquinolone antibacterial agents and structurally related amines with Goethite. Chemosphere, 2007, 66: 1502–1512
Hari AC, Paruchuri RA, Sabatini DA, Kibbey TC. Effects of pH and cationic and nonionic surfactants on the adsorption of pharmaceuticals to a natural aquifer material. Environ Sci Technol, 2005. 39: 2592–2598
Lorphensri O, Intravijit J, Sabatini DA, Kibbey TCG., Osathaphan K, Saiwan C. Sorption of acetaminophen, 17-ethynyl estradiol, nalidixic acid, and norfloxacin to silica, alumina, and a hydrophobic medium. Water Res, 2006, 40: 1481–1491
Zhang J, Li ZJ, Ge GF, Sun WC, Liang YC, Wu LS. Impacts of soil organic matter, pH and exogenous copper on sorption behavior of norfloxacin in three soils. J Environ Sci, 2009, 21, 632–640
Singh GD, Sharma R, Bawa AS, Saxena DC. Drying and rehydration characteristics of water chestnut (Trapa natans) as a function of drying air temperature. J Food Eng, 2008, 87: 213–221
Ross DL, Riley CM. Aqueous solubilities of some various substituted quinolone antimicrobials. Int J Pharm, 1990, 63: 237–250
Takaćs-Novák K, JÓzan M, Hermecz I, Szász G. Lipophilicity of antibacterial fluoroquinolones. Int J Pharm, 1992, 79: 89–96
Boehm HP. Some aspects of the surface chemistry of carbon blacks and other carbons. Carbon, 1994, 32: 759–769
Babic BM, Milonjic SK, Polovina MJ, Kaludierovic BV. Point of zero charge and intrinsic equilibrium constants of activated carbon cloth. Carbon, 1999, 37: 477–481
Gardea-Torresdey J, Becker-Hapak MK, Hosea JM, Darnall DW. Effect of chemical modification of algal carboxyl groups on metal ion binding. J Environ Sci Technol, 1990, 24: 1372–1378
Chen JP, Yang L. Study of a heavy metal biosorption onto raw and chemically modified Sargassum sp. via spectroscopic and modeling analysis. J Langmuir, 2006, 22: 8906–8914
Díaz-Díez MA, Gómez-Serrano V, Fernández-González C. Porous texture of activated carbons prepared by phosphoric acid activation of woods. Appl Surf Sci, 2004, 238: 309–313
Tessner CH, Vidic RD, Uranowski, LJ. Impact of oxygen-containing surface functional groups on activated carbon adsorption of phenols. Environ Sci Technol, 1997, 31: 1872–1878
Chen W, Duan L, Wang LL, Zhu DQ. Adsorption of hydroxyl- and amino-substituted aromatics to carbon manotubes. Environ Sci Technol, 2008, 42: 6862–6868
Meng G, Li A, Yang W, Liu F, Yang X, Zhang Q. Mechanism of oxidative reaction in the post crosslinking of hypercrosslinked polymers. Euro Polym J, 2007, 43: 2732–2737
Aroua MK, Leong SPP, Teo LY, Yin CY, Daud WMAW. Real-time determination of kinetics of adsorption of lead(II) onto palm shell-based activated carbon using ion selective electrode. J Bioresour Technol, 2008, 99: 5786–5792
Chingombe P, Saha B, Wakeman RJ. Sorption of atrazine on conventional and surface modified activated carbons. J Colloid Interf Sci, 2006, 302: 408–416
Weber WJ, Morriss JC. Kinetics of adsorption on carbon from solution. J Sanit Eng Div Am Soc Civil Eng. 1963, 89: 31–60
Gerçel Ö, Özcan A, Özcan AS, Gerçel HF. Preparation of activate carbon from a renewable bio-plant of Euphorbia rigida by H2SO4 activation and its adsorption behavior in aqueous solutions. Appl Surf Sci, 2007, 253: 4843–4852
Wu FC, Tseng RL, Juang RS. Comparisons of porous and adsorption properties of carbons activated by steam and KOH. J Coll Interf Sci, 2005, 283: 49–56
Langmuir I. The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc, 1918, 40: 1361–1403
Kubilay S, Gurkan R, Savran A, Sahan T. Removal of Cu(II), Zn(II) and Co(II) ions from aqueous solutions by adsorption onto natural bentonite. Adsorption, 2007, 13: 41–51
Freundlich H. Adsorption in solution. J Phys Chem Soc, 1906, 40: 1361–1368
Crini G, Peindy HN, Gimbert F, Robert C. Basic Green 4 (Malachite Green) from aqueous solutions by adsorption using cyclodextrin-based adsorbent: kinetic and equilibrium studies. Sep Purif Technol, 2007, 53: 97–110
Kavitha D, Namasivayam C. Experimental and kinetic studies on methylene blue adsorption by coir pith carbon. Bioresour Technol, 2007, 98: 14–21
Aharoni C, Ungarish M. Kinetics of activated chemisorption. Part 2. Theoretical models. J Chem Soc Faraday Trans, 1977, 73: 456–464
Yu XQ, Zipp GL, Davidson GWR III. The effect of temperature and pH on the solubility of quinolone compounds: estimation of heat of fusion. Pharm Res, 1994, 11: 522–527
Pan B, Xing BS. Adsorption mechanisms of organic chemicals on carbon nanotubes. Environ Sci Technol, 2008, 42: 9005–9013
Ross DL, Riley CM. Dissociation and complexation of the fluoroquinolone antimicrobials—An update. J Pharm Biomed Anal, 1994, 12: 1325–1331
Author information
Authors and Affiliations
Corresponding author
Additional information
equal contribution to this work
Rights and permissions
About this article
Cite this article
Xie, H., Liu, W., Zhang, J. et al. Sorption of norfloxacin from aqueous solutions by activated carbon developed from Trapa natans husk. Sci. China Chem. 54, 835–843 (2011). https://doi.org/10.1007/s11426-010-4132-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-010-4132-7