Abstract
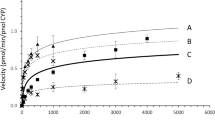
The cytochrome P450 (CYP) superfamily plays a key role in the oxidative metabolism of a wide range of exogenous chemicals. CYP2C8 is the principal enzyme responsible for the metabolism of the anti-cancer drug paclitaxel in the human liver, and carries out the oxidative metabolism of at least 5% of clinical drugs. Polymorphisms in CYP2C8 have been closely implicated in individualized medication. CYP2C8.3, a common polymorph of CYP2C8 with dual amino acid substitutions R139K and K399R, is found primarily in Caucasians. In this study, CYP2C8.3 and its wild type (WT) CYP2C8 were expressed in E. coli, and their purified proteins were characterized by UV-visible spectroscopy, mass spectrometry, and circular dichroism. Their thermal stability, substrate binding ability, and metabolic activity against paclitaxel were investigated. The electron transfer kinetics during paclitaxel metabolism by WT CYP2C8 or CYP2C8.3 was studied by stopped-flow kinetics. The results revealed that mutations in CYP2C8.3 did not greatly influence the heme active site or protein thermal stability and paclitaxel binding ability, but the metabolic activity against paclitaxel was significantly depressed to just 11% of that of WT CYP2C8. Electron transfer from CYP reductase to CYP2C8.3 was found to be significantly slower than that to WT CYP2C8 during catalysis, and this might be the main reason for the depressed metabolic activity. Since the polymorph CYP2C8.3 is defective in catalyzing substrates of CYP2C8 in vitro, it might be expected to have important clinical and pathophysiological consequences in homozygous individuals, and this study provides valuable information in this aspect.
Similar content being viewed by others
References
Plasilova M, Stoilov I, Sarfarazi M, Kadasi L, Ferakova E, Ferak V. Identification of a single ancestral CYP1B1 mutation in Slovak Gypsies (Roms) affected with primary congenital glaucoma. J Med Genet, 1999, 36: 290–294
Stoilov I, Akarsu AN, Alozie I, Child A, Barsoum-Homsy M, Turacli ME. Sequence analysis and homology modeling suggest that primary congenital glaucoma on 2p21 results from mutations disrupting either the hinge region or the conserved core structures of cytochrome P4501B1. Am J Hum Genet, 1998, 62: 573–584
Shimada T, Yamazaki H, Mimura M, Inui Y, Guengerich FP. Interindividual variations in human liver cytochrome-P450 enzymes involved in the oxidation of drugs, carcinogens and toxic-chemicals-studies with liver-microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther, 1994, 270: 414–423
Rendic S, DiCarlo FJ. Human cytochrome P450 enzymes: A status report summarizing their reactions, substrates, inducers, and inhibitors. Drug Metab Rev, 1997, 29: 413–580
Totah RA, Rettie AE. Cytochrome P450 2C8: Substrates, inhibitors, pharmacogenetics, and clinical relevance. Clin Pharmacol Ther, 2005, 78: 153–153
Schoch GA, Yano JK, Wester MR, Griffin KJ, Stout CD, Johnson EF. Structure of human microsomal cytochrome P4502C8—Evidence for a peripheral fatty acid binding site. J Biol Chem, 2004, 279: 9497–9503
Rahman A, Korzekwa KR, Grogan J, Gonzalez FJ, Harris JW. Selective biotransformation of taxol to 6-α-hydroxytaxol by human cytochrome-P450 2C8. Cancer Res, 1994, 54: 5543–5546
Sonnichsen DS, Liu Q, Schuetz EG, Schuetz JD, Pappo A, Relling MV. Variability in human cytochrome-P450 paclitaxel metabolism. J Pharmacol Exp Ther, 1995, 275: 566–5759
Ohyama K, Nakajima M, Nakamura S, Shimada N, Yamazaki H, Yokoi T. A significant role of human cytochrome P4502C8 in amio darone N-deethylation: An approach to predict the contribution with relative activity factor. Drug Metab Dispos, 2000, 28: 1303–1310
Yamazaki H, Shibata A, Suzuki M, Nakajima M, Shimada N, Guengerich FP, Yokoi T. Oxidation of troglitazone to a quinone-type metabolite catalyzed by cytochrome P-4502C8 and P-450 3A4 in human liver microsomes. Drug Metab Dispos, 1999, 27: 1260–1266
Kerr BM, Thummel KE, Wurden CJ, Klein SM, Kroetz DL, Gonzalez FJ, Levy RH. Human liver carbamazepine metabolism—role of CYP3A4 and CYP2C8 in 10,11-epoxide formation. Biochem Pharmacol, 1994, 47: 1969–1979
Muck W. Clinical pharmacokinetics of cerivastatin. Clin Pharmacokinet, 2000, 39: 99–116
Yasar U, Bennet AM, Eliasson E, Lundgren S, Wiman B, de Faire U, Rane A. Allelic variants of cytochromes P4502C modify the risk for acute myocardial infarction. Pharmacogenetics, 2003, 13: 715–720
Ishikawa C, Ozaki H, Nakajima T, Ishii T, Kanai S, Anjo S, Shirai K, Inoue I. A frameshift variant of CYP2C8 was identified in a patient who suffered from rhabdomyolysis after administration of cerivastatin. J Hum Genet, 2004, 49: 582–585
Dai D, Zeldin DC, Blaisdell JA. Chanas B, Coulter SJ, Ghanayem BI, Goldstein JA. Polymorphisms in human CYP2C8 decrease me tabolism of the anticancer drug paclitaxel and arachidonic acid. Pharmacogenetics, 2001, 11: 597–607
Soyama A, Hanioka N, Saito Y, Murayama N, Ando M, Ozawa S, Sawada J. Amiodarone N-deethylation by CYP2C8 and its variants, CYP2C8*3 and CYP2C8 P404A. Pharmacol Toxicol, 2002, 91: 174–178
Bahadur N, Leathart JBS, Mutch E, Dorothy SC, Dunn SA, Gilissen R, Houdt JV, Hendrickx J, Mannens G, Bohets H, Williams FM, Armstrong M, Crespi CL, Daly AK. CYP2C8 polymorphisms in Caucasians and their relationship with paclitaxel 6α-hydroxylase activity in human liver microsomes. Biochem Pharmacol, 2002, 64: 1579–1589
Hichiya H, Tanaka-Kagawa T, Soyama A, Jinno H, Koyano S, Katori N, Matsushima E, Uchiyama S, Tokunaga H, Kimura H, Minami N, Katoh M, Sugai K, Goto Y, Tamura T, Yamamoto N, Ohe Y, Kunitoh H, Nokihara H, Yoshida T, Minami H, Saijo N, Ando M, Ozawa S, Saito Y, Sawada J. Functional characterization of five novel CYP2C8 variants, G171S, R186X, R186G, K247R, and K383N, found in a Japanese population. Drug Metab Dispos, 2005, 33: 630–636
Wester MR, Stout CD, Johnson EF. Purification and crystallization of N-terminally truncated forms of microsomal cytochrome P450 2C5. Methods Enzymol, 2002, 357: 73–79
Wester MR, Yano JK, Schoch GA, Yang C, Griffin KJ, Stout CD, Johnson EF. The structure of human cytochrome P450 2C9 complexed with flurbiprofen at 2.0-Å resolution. J Biol Chem, 2004, 279: 35630–35637
Berry EA, Trumpower BL. Simultaneous determination of hemes a, b and c from pyridine hemochrome spectra. Anal Biochem, 1987, 161: 1–15
Omura T, Sato R. Carbon monoxide-binding pigment of liver microsomes. Evidence for its hemoprotein nature. J Biol Chem, 1964, 239: 2370–2378
Omura T, Sato R. Carbon monoxide-binding pigment of liver microsomes. II Solubilization purification and properties. J Biol Chem, 1964, 239: 2379–2385
Chen CD, Doray B, Kemper B. Efficient assembly of functional cytochrome P450 2C2 requires a spacer sequence between the N-terminal signal anchor and catalytic domains. J Biol Chem, 1997, 272: 22891–22897
Jwexpl32. Version 1.54.03 [Build 1]. Japan: JASCO Corporation
Dickmann LJ, Locuson CW, Jones JP, Rettie AE. Differential roles of Arg97, Asp293, and Arg108 in enzyme stability and substrate specificity of CYP2C9. Mol Pharmacol, 2004, 65: 842–850
Richardson TH, Jung F, Griffin KJ, Wester M, Raucy JL, Kemper B, Bornheim LM, Hassett C, Omiecinski CJ, Johnson EF. Universal approach to the expression of human and rabbit cytochrome P450S of the 2C subfamily in Escherichia-coli. Arch Biochem Biophys, 1995, 323: 87–96
Sun L, Wang ZH, Ni FY, Tan XS, Huang ZX. The role of Ile476 in the structural stability and substrate binding of human cytochrome P450 2C8. Protein J, 2010, 29: 32–43
Martinis SA, Blanke SR, Hager LP, Sligar SG, Hoa GHB, Rux JJ, Dawson JH. Probing the heme iron coordination structure of pressure-induced cytochrome P420(cam). Biochemistry, 1996, 35: 14530–14536
Denisov IG, Makris TM, Sligar SG, Schlichting I. Structure and chemistry of cytochrome P450. Chem Rev, 2005, 105: 2253–2277
Haugen DA, Coon MJ. Properties of electrophoretically homogeneous phenobarbital-inducible and β-naphthoflavone-inducible forms of liver microsomal cytochrome -P-450. J Biol Chem, 1976, 251: 7929–7939
Dawson JH, Andersson LA, Sono M. Spectroscopic investifigations of ferric cytochrome P-450-cam ligand complexes—identification of the ligand trans to cysteinate in the native enzyme. J Biol Chem, 1982, 257: 3606–3617
Mclean MA, Maves SA, Weiss KE, Krepich S, Sligar SG. Characterization of a cytochrome P450 from the acidothermophilic archaea Sulfolobus solfataricus. Biochem Biophys Res Commun, 1998, 252: 166–172
Koo LS, Tschirret-Guth RA, Straub WE, Moenne-Loccoz P, Loehr TM, Ortiz de Montellano PR. The active site of the thermophilic CYP119 from Sulfolobus solfataricus. J Biol Chem, 2000, 275: 14112–14123
Wang HP, Kimura T. Purification and characterization of adrenal cortex mitochondrial cytochrome P-450 specific for cholesterol side chain cleavage activity. J Biol Chem, 1976, 251: 6068–6074
Andersson LA, Peterson JA. Active-site analysis of ferric P450 enzymes: hydrogen-bonding effects on the circular-dichroism spectra. Biochem Biophys Res Commun, 1995, 211: 389–395
Jefcoate CR. Measurement of substrate and inhibitor binding to microsomal cytochrome P-450 by optical-difference spectroscopy. Methods Enzymol, 1978, 52: 258–279
Jung F, Griffin KJ, Song W, Richardson TH, Yang M, Johnson EF. Dentification of amino acid substitutions that confer a high affinity for sulfaphenazole binding and a high catalytic efficiency for warfarin metabolism to P450 2C19. Biochemistry, 1998, 37: 16270–16279
Koo LS, Immoos CE, Cohen MS, Farmer PJ, Ortiz de Montellano PR. Enhanced electron transfer and lauric acid hydroxylation by site-directed mutagenesis of CYP119. J Am Chem Soc, 2002, 124: 5684–5691
Scott EE, White MA, He YA, Johnson EF, Stout CD, Halpert JR. Structure of mammalian cytochrome P4502B4 complexed with 4-(4-chlorophenyl) imidazole at 1.9-Å resolution—Insight into the range of P450 conformations and the coordination of redox partner binding. J Biol Chem, 2004, 279: 27294–27301
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Jiang, H., Sun, L., Huang, Z. et al. Structural and functional insights into CYP2C8.3: A genetic polymorph of cytochrome P450 2C8. Sci. China Chem. 53, 2200–2207 (2010). https://doi.org/10.1007/s11426-010-4087-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-010-4087-8