Abstract
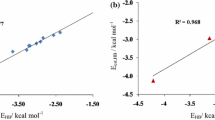
The individual hydrogen bonding energies in N-methylacetamide chains were evaluated at the MP2/6-31+G** level including BSSE correction and at the B3LYP/6-311++G(3df,2pd) level including BSSE and van der Waals correction. The calculation results indicate that compared with MP2 results, B3LYP calculations without van der Waals correction underestimate the individual hydrogen bonding energies about 5.4 kJ mol−1 for both the terminal and central hydrogen bonds, whereas B3LYP calculations with van der Waals correction produce almost the same individual hydrogen bonding energies as MP2 does for those terminal hydrogen bonds, but still underestimate the individual hydrogen bonding energies about 2.5 kJ mol−1 for the hydrogen bonds near the center. Our calculation results show that the individual hydrogen bonding energy becomes more negative (more attractive) as the chain becomes longer and that the hydrogen bonds close to the interior of the chain are stronger than those near the ends. The weakest individual hydrogen bonding energy is about −29.0 kJ mol−1 found in the dimer, whereas with the growth of the N-methylacetamide chain the individual hydrogen bonding energy was estimated to be as large as −62.5 kJ mol−1 found in the N-methylacetamide decamer, showing that there is a significant hydrogen bond cooperative effect in N-methylacetamide chains. The natural bond orbital analysis indicates that a stronger hydrogen bond corresponds to a larger positive charge for the H atom and a larger negative charge for the O atom in the N-H⋯O=C bond, corresponds to a stronger second-order stabilization energy between the oxygen lone pair and the N-H antibonding orbital, and corresponds to more charge transfer between the hydrogen bonded donor and acceptor molecules.
Similar content being viewed by others
References
Jerffery GA. An Introduction to Hydrogen Bonding. New York: Oxford University Press, 1997. 1–2
Scheiner S. Hydrogen Bonding: A Theoretical Perspective. New York: Oxford University Press, 1997. 11–14
Kang YK. Which functional form is appropriate for hydrogen bond of amides. J Phys Chem B, 2000, 104: 8321–8326
Wang CS, Zhang Y, Gao K, Yang ZZ. A new scheme for determining the intramolecular seven-membered ring N-H⋯O=C hydrogen-bonding energies of glycine and alanine peptides. J Chem Phys, 2005, 123: 024307
Yang Y. Theoretical study of the S-H⋯O blue-shifted hydrogen bond. Int J Quantum Chem, 2009, 109: 266–274
Zhang Y, Wang CS, Yang ZZ. Estimation on the interamolcular 8- and 12-membered ring N-H⋯O=C hydrogen bonding energies in β-peptides. J Theor Comput Chem, 2009, 8: 279–297
Deshmukh MM, Bartolotti LJ, Gadre SR. Intramolecular hydrogen bonding and cooperative interactions in carbohydrates via the molecular tailoring approach. J Phys Chem A, 2008, 112: 312–321
Jiang XN, Sun CL, Wang CS. A scheme for rapid prediction of cooperativity in hydrogen bond chains of formamides, acetamides, and N-methylformamides. J Comput Chem, 2010, 31: 1410–1420
Kobko N, Paraskevas L, del Rio E, Dannenberg JJ. Cooperativity in amide hydrogen bonding chains: Implications for protein-folding models. J Am Chem Soc, 2001, 123: 4348–4349
Kobko N, Dannenberg JJ. Cooperativity in amide hydrogen bonding chains. Relation between energy, position, and H-bond chain length in peptide and protein folding models. J Phys Chem A, 2003, 107: 10389–10395
Kobko N, Dannenberg JJ. Cooperativity in amide hydrogen bonding chains. A comparison between vibrational coupling through hydrogen bonds and covalent bonds. Implications for peptide vibrational spectra. J Phys Chem A, 2003, 107: 6688–6697
Deshmukh MM, Gadre SR. Estimation of N-H⋯O=C intramolecular hydrogen bond energy in polypeptides. J Phys Chem A, 2009, 113: 7927–7932
Kennedy RJ, Tsang KY, Kemp DS. Consistent helicities from CD and template t/c data for N-templated polyalanines: Progress toward resolution of the alanine helicity problem. J Am Chem Soc, 2002, 124: 934–944
Tan HW, Qu WW, Chen GJ, Liu RZ. The role of charge transfer in the hydrogen bond cooperative effect of cis-N-methylformamide oligomers. J Phys Chem A, 2005, 109: 6303–6308
Wang ZX, Wu C, Lei HX, Duan Y. Accurate ab initio study on the hydrogen-bond pairs in protein secondary structures. J Chem Theory Comput, 2007, 3: 1527–1537
Asensio A, Kobko N, Dannenberg JJ. Cooperative hydrogen-bonding in adenine-thymine and guanine-cytosine base pairs. Density functional theory and Møller-Plesset molecular orbital study. J Phys Chem A, 2003, 107: 6441–6443
Ludwig R, Weinhold F, Farrar TC. Structure of liquid N-methylacetamide: Temperature dependence of NMR chemical shifts and quadrupole coupling constants. J Phys Chem A, 1997, 101: 8861–8870
Huelsekopf M, Ludwig R. Correlations between structural, NMR and IR spectroscopic properties of N-methylacetamide. Magn Reson Chem, 2001, 39: S127–S134
Sun CL, Jiang XN, Wang CS. An analytic potential energy function for the amide-amide and amide-water intermolecular hydrogen bonds in peptides. J Comput Chem, 2009, 30: 2567–2575
Ireta J, Neugebauer J, Scheffler M, Rojo A, Galvan M. Density functional theory study of the cooperativity of hydrogen bonds in finite and infinite α-helices. J Phys Chem B, 2003, 107: 1432–1437
Scheiner S. Contributions of NH⋯O and CH⋯O hydrogen bonds to the stability of β-sheets in proteins. J Phys Chem B, 2006, 110: 18670–18679
Møller C, Plesset MS. Note on an approximation treatment for many-electron systems. Phys Rev, 1934, 46: 618–622
Becke AD. Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys, 1993, 98: 5648–5652
Lee C, Yang W, Parr TG. Development of the colle-salvetti correlation-energy formula into a functional of the electron density. Phys Rev B, 1988, 37: 785–789
Rao L, Ke H, Fu G, Xu X, Yan Y. Performance of several density functional theory methods on describing hydrogen-bond interactions. J Chem Theory Comput, 2009, 5: 86–96
Halgren TA. Representation of van der Waals (vdW) interactions in molecular mechanics force fields: Potential form, combination rules, and vdW parameters. J Am Chem Soc, 1992, 114: 7827–7843
Wang J, Cieplak P, Kollman PA. How well does a restrained electrostatic potential (RESP) model perform in calculating conformational energies of organic and biological molecules. J Comput Chem, 2000, 21: 1049–1074
Cornell WD, Cieplak P, Bayly CI, Gould IR, Merz KM, Ferguson DM, Spellmeyer DC, Fox T, Caldwell JW, Kollman PA. A second generation force field for the simulation of proteins, nucleic acids, and organic molecules. J Am Chem Soc, 1995, 117: 5179–5197
Moyna G, Williams HJ, Nachman RJ, Scott AI. Conformation in solution and dynamics of a structurally constrained linear insect kinin pentapeptide analogue. Biopolymers, 1999, 49: 403–413
Ross WS, Hardin CC. Ion-induced stabilization of the G-DNA quadruplex: Free energy perturbation studies. J Am Chem Soc, 1994, 116: 6070–6080
Aquist J. Ion-water interaction potentials derived from free energy perturbation simulations. J Phys Chem, 1990, 94: 8021–8024
Boys SF, Bernardi F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys, 1970, 19: 553–566
Simon S, Duran M, Dannenberg JJ. How does basis set superposition error change the potential surfaces for hydrogen-bonded dimers. J Chem Phys, 1996, 105: 11024–11031
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA. Gaussian 03. Revision B.02. Pittsburgh (PA): Gaussian Inc. 2003
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiang, X., Wang, C. Evaluation of the individual hydrogen bonding energies in N-methylacetamide chains. Sci. China Chem. 53, 1754–1761 (2010). https://doi.org/10.1007/s11426-010-4047-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-010-4047-3