Abstract
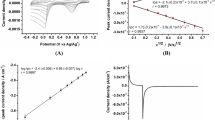
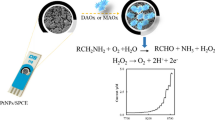
The screen-printed three-electrode system was applied to fabricate a new type of disposable amperometric xanthine oxidase biosensor. Carbon-working, carbon-counter and Ag/AgCl reference electrodes were all manually printed on the polyethylene terephthalate substrate forming the screen-printed three-electrode system by the conventional screen-printing process. As a mediator, Prussian blue could not only catalyze the electrochemical reduction of hydrogen peroxide produced from the enzyme reaction, but also keep the favorable potential around 0 V. The optimum operational conditions, including pH, potential and temperature, were investigated. The sensitivities of xanthine and hypoxanthine detections were 13.83 mA/M and 25.56 mA/M, respectively. A linear relationship was obtained in the concentration range between 0.10 μM and 4.98 μM for xanthine and between 0.50 μM and 3.98 μM for hypoxanthine. The small Michaelis-menten constant value of the xanthine oxidase biosensor was calculated to be 3.90 μM. The results indicate that the fabricated xanthine oxidase biosensor is effective and sensitive for the detection of xanthine and hypoxanthine.
Similar content being viewed by others
References
Lindberg D, Grimes C. The effect of trade globalization on food safety. Sensor Lett, 2004, 2: 161–163
Hu S, Xu C, Luo J, Luo J, Cui D. Biosensor for detection of hypoxanthine based on xanthine oxidase immobilized on chemically modified carbon paste electrode. Anal Chim Acta, 2000, 412: 55–61
Sugase S, Tsuda T. Determination of lactic acid, uric acid, xanthine and tyrosine in human sweat by HPLC, and the concentration variation of lactic acid in it after the intake of wine. Bunseki Kagaku, 2002, 51: 429–435
Samanidou VF, Metaxa AS, Papadoyannis IN. Direct simultaneous determination of uremic toxins: Creatine, creatinine, uric acid, and xanthine in human biofluids by HPLC. J Liq Chromatogr Relat Technol, 2002, 25: 43–57
Chan ST, Yao MWY, Wong YC, Wong T, Mok CS, Sin DWM. Evaluation of chemical indicators for monitoring freshness of food and determination of volatile amines in fish by headspace solid-phase microextraction and gas chromatography-mass spectrometry. Eur Food Res Technol, 2006, 224: 67–74
Olafsdottir G, Martinsdottir E, Ohelenschlager J. Methods to evaluate fish freshness in research and industry. Trends Food Sci Technol, 1997, 8: 258–265
Armenta S, Coelho NMM, Roda R, Garrigues S, de la Guardia M. Seafood freshness determination through vapour phase Fourier transform infrared spectroscopy. Anal Chim Acta, 2006, 580: 216–222
Smulevich G, Droghetti E, Focardi C, Coletta M, Ciaccio C, Nocentini M. A rapid spectroscopic method to detect the fraudulent treatment of tuna fish with carbon monoxide. Food Chem, 2007, 101: 1071–1077
Chen XM, Lin ZJ, Chen DJ, Jia TT, Cai ZM, Wang XR, Chen X, Chen GN, Oyama M. Nonenzymatic amperometric sensing of glucose by using palladium nanoparticles supported on functional carbon nanotubes. Biosens Bioelectron, 2010, 25: 1803–1808
Yuan JH, Wang K, Xia XH. Highly ordered platinum-nanotubule arrays for amperometric glucose sensing. Adv Func Mater, 2005, 15: 803–809
Venugopal V. Biosensors in fish production and quality control. Biosens Bioelectron, 2002, 17: 147–157
Kirgoz OA, Timur S, Wang J, Telefoncu A. Xanthine oxidase modified glassy carbon paste electrode. Electrochem Commun, 2004, 6: 913–916
Hoshi T, Noguchi T, Anzai J. The preparation of amperometric xanthine sensors based on multilayer thin films containing xanthine oxidase. Mater Sci Eng C, 2006, 26: 100–103
Cubukcu M, Timur S, Anik U. Examination of performance of glassy carbon paste electrode modified with gold nanoparticle and xanthine oxidase for xanthine and hypoxanthine detection. Talanta, 2007, 74: 434–439
Li XH, Xie ZH, Min H, Xian YZ, Jin LT. Amperometric biosensor for hypoxanthine based on immobilized xanthine oxidase on iron (III) meso-tetraphenylporphyrin nanoparticles modified glassy carbon electrode. Anal Lett, 2008, 41: 456–468
Zhang M, Li L, Du Z, Xu S, Li C, Chen X, Zhang T, Wang T. Fast response amperometric biosensor for H2O2 detection based on horse-radish-peroxidase/titania-nanowires/chitosan modified glassy carbon electrode. Sensor Lett, 2009, 7: 543–549
Ignatov S, Shishniashvili D, Ge B, Scheller FW, Lisdat F. Amperometric biosensor based on a functionalized gold electrode for the detection of antioxidants. Biosens Bioelectron, 2002, 17: 191–199
Wang K, Chen JH, Chen J, Liu AL, Li GW, Luo HB, Lin XH, Chen YZ. A sandwich-type electrochemical biosensor for detection of bcr/abl fusion gene using locked nucleic acids on gold electrode. Electroanal, 2009, 21: 1159–1166
Sun W, Gao R, Jiao K. Electrochemistry and electrocatalysis of hemoglobin in nafion/nano-CaCO3 film on a new ionic liquid BPPF6 modified carbon paste electrode. J Phys Chem B, 2007, 111: 4560–4567
Mashhadizadeh MH, Talakesh M, Peste M, Momeni A, Hamidian H, Mazlum M. A novel modified carbon paste electrode for potentiometric determination of mercury(II) ion. Electroanalysis, 2006, 18: 2174
Xue MH, Xu Q, Zhou M, Zhu JJ. In situ immobilization of glucose oxidase in chitosan-gold nanoparticle hybrid film on Prussian Blue modified electrode for high-sensitivity glucose detection. Electrochem Commun, 2006, 8: 1468–1474
Chen HL, Zhao L, Chen X, Zhuang ZX, Wang XR. Development of an amperometric glucose biosensor based on the immobilization of glucose oxidase in an ormosil-PVA matrix onto a Prussian Blue modified electrode. Sci China Ser-B: Chem, 2009, 52: 1128–1135
Suprun E, Evtugyn G, Budnikov H, Ricci F, Moscone D, Palleschi G. Acetylcholinesterase sensor based on screen-printed carbon electrode modified with Prussian blue. Anal Bioanal Chem, 2005, 383: 597–604
Lowinsohn D, Bertotti M. Flow injection analysis of blood l-lactate by using a Prussian Blue-based biosensor as amperometric detector. Anal Biochem, 2007, 365: 260–265
Karyakin AA, Gitelmacher OV, Karyakina EE. Prussian blue-based first-generation biosensor. A sensitive amperometric electrode for glucose. Anal Chem, 1995, 67: 2419–2423
Wang J, Pedrero M, Sakslund H, Hammerich O, Pingarron J. Electrochemical activation of screen-printed carbon strips. Analyst, 1996, 121: 345–350
de Mattos IL, Gorton L, Laurell T, Malinauskas A, Karyakin AA. Development of biosensors based on hexacyanoferrates. Talanta, 2000, 52: 791–799
de Mattos IL, Gorton L, Ruzgas T. Sensor and biosensor based on Prussian Blue modified gold and platinum screen printed electrodes. Biosens Bioelectron, 2003, 18: 193–200
Li M, Zhao G, Yue Z, Huang S. Sensor for traces of hydrogen peroxide using an electrode modified by multiwalled carbon nanotubes, a gold-chitosan colloid, and Prussian blue. Microchimica Acta, 2009, 167: 167–172
Aymard C, Belarbi A. Kinetics of thermal deactivation of enzymes: a simple three parameters phenomenological model can describe the decay of enzyme activity, irrespectively of the mechanism. Enzyme Microb Technol, 2000, 27: 612–618
Villalonga R, Matos M, Cao R. Construction of an amperometric biosensor for xanthine via supramolecular associations Electrochem Commun, 2007, 9: 454–458
Liu Y, Nie L, Tao W, Yao S. Amperometric study of Au-colloid function on xanthine biosensor based on xanthine oxidase immobilized in polypyrrole layer. Electroanalysis, 2004, 16: 1271–1278
Shan D, Wang YN, Xue HG, Cosnier S, Ding SN. Xanthine oxidase/laponite nanoparticles immobilized on glassy carbon electrode: Direct electron transfer and multielectrocatalysis. Biosens Bioelectron, 2009, 24: 3556–3561
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Teng, Y., Chen, C., Zhou, C. et al. Disposable amperometric biosensors based on xanthine oxidase immobilized in the Prussian blue modified screen-printed three-electrode system. Sci. China Chem. 53, 2581–2586 (2010). https://doi.org/10.1007/s11426-010-4038-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-010-4038-4