Abstract
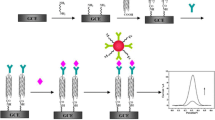
The novel nanocomposites were obtained by covalently linking arginine enantiomers with the sidewall of multi-walled carbon nanotubes, and utilized as sensing materials for biomolecular recognition in electrochemical immunoassay. They were characterized by X-ray photoelectron spectroscopy (XPS), transmission electron microscopy (TEM) and circular dichroism spectroscopy (CD). Nano-gold and Prussian Blue were employed to amplify the analytical signal responses. Prostate specific antibody/antigen and carcinoembryonic antibody/antigen were used as model systems. The results showed that the different configurations of nanocomposites could readily facilitate chiral recognition of antibodies, and D-nanocomposites displayed a larger signal.
Similar content being viewed by others
References
Tang K, Gan H, Li Y, Chi L, Sun T, Fuchs H. Stereoselective interaction between DNA and chiral surfaces. J Am Chem Soc, 2008, 130: 11284–11285
Sun T, Han D, Rhemann K, Chi L, Fuchs H. Stereospecific interaction between immune cells and chiral surfaces. J Am Chem Soc, 2007, 129: 1496–1497
Hazen RM, Sholl DS. Chiral selection on inorganic crystalline surfaces. Nat Mater, 2003, 2: 367–374
Stupp SI, LeBonheur V, Walker K., Li LS, Huggins KE, Keser M, Amstutz A. Supramolecular materials: Self-organized nanostructures. Science. 1997, 276: 384–389
Cornelissen JJLM, Rowan AE, Nolte RJM, Sommerdijk NAJM. Chiral architectures from macromolecular building blocks. Chem Rev, 2001, 101: 4039–4070
Cui X, Fu YZ, Li MS, Chen M, He X, Liu XH, Feng XM. Application of L-thiazolidine-4-carboxylic acid monolayer in electrochemical determination of copper (II). Sci China Chem, 2010, 1: 257–262
Izake EL. Chiral discrimination and enantioselective analysis of drugs: an overview. J Pharm Sci, 2007, 96:1659–1676
Wang WH, Rusin O, Xu XY, Kim KK, Escobedo JO, Fakayode SO, Fletcher KA, Lowry M, Schowalter CM, Lawrence CM, Fronczek FR, Warner IM, Strongin RM. Detection of homocysteine and cysteine. J Am Chem Soc, 2005, 127: 15949–15958
Leitner A, Lindner W. Functional probing of arginine residues in proteins using mass spectrometry and an arginine-specific covalent tagging concept. Anal Chem, 2005, 77: 4481–4488
Schug KA, Lindner W. Noncovalent binding between guanidinium and anionic groups: Focus on biological- and synthetic-based arginine/guanidinium interactions with phosphate and sulfate residues. Chem Rev, 2005, 105: 67–113
Caldwell J. Stereochemical determinants of the nature and consequences of drug metabolism. J Chromatogr, 1995, A694: 39–48
Eichelbaum M, Cross AS. Stereochemical aspects of drug action and disposition. Adv Drug Res, 1996, 28: 1–64
Nakamura H, Saheki T, Nakagawa S. Differential cellular localization of enzymes of L-arginine metabolism in the rat brain. Brain Res, 1990, 530: 108–112
Li G, Regunathan S, Barrow CJ, Eshraghi J, Cooper R, Reis DJ. Agmatine is an endogenous clonidine-displacing substance in brain. Science, 1994, 263: 966–969
Calver A, Collier J, Vallance P. Dilator actions of arginine in human peripheral vasculature. Clin Sci (Lond), 1991, 81: 695–700
Warsinke A, Benkert A, Scheller, Fresen FW. Electrochemical immunoassays. J Anal Chem, 2000, 366: 622–634
Bilitewski U. Peer reviewed: Can affinity sensors be used to detect food contaminants. Anal Chem, 2000, 72:692A–701A
Van Emon JM, Lopez-Avila V. Immunochemical methods for environmental analysis. Anal Chem, 1992, 64: 79A–88A
Attard GA. Electrochemical studies of enantioselectivity at chiral metal surfaces. J Phy Chem B, 2001, 105:3158–3167
Stefan RI, Van Staden JF, Aboul-Enein HY. Molecular recognition in chiral discrimination. Cryst Eng, 2001, 4: 113–118
Zhang H, Li DQ, Shao GS, Yuan ZY. A simple method to prepare titania nanomaterials of core-shell structure, hollow nanospheres and mesoporous nanoparticles. Sci China Ser B Chem, 2009, 9: 1498–1503
Liu BL, Xiao HP, Song Y, You XZ. Two bimetallic W(IV)-Mn(II) complexes based on octacyanometallates: Structures and magnetic properties. Sci China Ser B Chem, 2009, 11: 1801–1807
Ren YR, Qu MZ, Yu ZL. SiO/CNTs: A new anode composition for lithium-ion battery. Sci China Ser B Chem, 2009, 12: 2047–2050
Heller A. Electrical wiring of redox enzymes. Acc Chem Res, 1990, 23: 128–134
Moore RR, Banks CE, Compton RG. Basal plane pyrolytic graphite modified electrodes: Comparison of carbon nanotubes and graphite powder as electrocatalysts. Anal Chem, 2004, 76: 2677–2682
Gong KP, Zhang MN, Yan YM, Su L, Mao LQ, Xiong SX, Chen Y. Sol-gel-derived ceramic-carbon nanotube nanocomposite electrodes: Tunable electrode dimension and potential electrochemical applications. Anal Chem, 2004, 76: 6500–6505
Martin E W, Kibbey W E, DiVecchia L, Anderson G, Catalano P, Minton JP. Carcinoembryonic antigen: Clinical and historical aspects. Cancer, 1976, 37: 62–81
Guo ML, Chen JH, Nie LH, Yao SZ. Electrostatic assembly of calf thymus DNA on multi-walled carbon nanotube modified gold electrode and its interaction with chlorpromazine hydrochloride. Electrochim Acta, 2004, 49: 2637–2643
Finot MO, Braybrook GD, Mcdermott MT. Characterization of electrochemically deposited gold nanocrystals on glassy carbon electrodes. J Electroanal Chem, 1999, 466: 234–241
Zhuo Y, Yuan PX, Yuan R, Chai YQ, Hong CL. Nanostructured conductive material containing ferrocenyl for reagentless amperometric immunosensors. Biomaterials, 2008, 29: 1501–1508
Zakharchuk NF, Meyer B, Henning H, Scholz F, Jaworksi A, Stojek Z. A comparative study of Prussian-Blue-modified graphite paste electrodes and solid graphite electrodes with mechanically immobilized Prussian Blue. J Electroanal Chem, 1995, 398: 23–35
Lupu S, Mihailciuc C, Pigani L, Seeber R, Totir N, Zanardi C. Electrochemical preparation and characterisation of bilayer films composed by Prussian Blue and conducting polymer. Electrochem Commun, 2002, 4: 753–758
Yu H, Sheng QL, Li L, Zheng JB. Rapid electrochemical preparation of a compact and thick Prussian Blue film on composite ceramic carbon electrode from single ferricyanide solution in the presence of HAuCl4. J Electroanal Chem, 2007, 606: 55–62
Pyrasch M, Toutianoush A, Jin WQ, Schnepf J, Tieke B. Self-assembled films of Prussian Blue and analogues: Optical and electrochemical properties and application as ion-sieving membranes. Chem Mater, 2003, 15: 245–254
Liu Y, Chu ZY, Jin WQ. A sensitivity-controlled hydrogen peroxide sensor based on self-assembled Prussian Blue modified electrode. Electrochem Commun, 2009, 11: 484–487
Booth TD, Wahnon D, Wainer IW. Is chiral recognition a three-point process? Chirality, 1997, 9: 96–98
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fu, Y., Chen, M., Cui, X. et al. Recognition behavior of chiral nanocomposites toward biomolecules and its application in electrochemical immunoassay. Sci. China Chem. 53, 1453–1458 (2010). https://doi.org/10.1007/s11426-010-4011-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-010-4011-2