Abstract
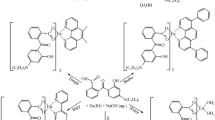
A novel strong-fluorescent Eu-containing hydrotalcite-like compound (Eu-HTlc) was synthesized using the coprecipitation method, in which aluminum(III) was partially substituted by Europium (III) in the hydrotalcite-like layers, and thenoyltrifluoroacetone, 1,10-phenanthroline were dispersed into the anions in the interlayer region. The sample was characterized by XRD, XPS, FT-IR, ICP, TG-DSC, TEM and fluorescence spectra, and its composition and structure were determined. The results indicated that the sample exhibited a characteristic red light (614 nm). The fluorescent lifetime and fluorescence quantum yield of Eu-HTlc were measured to be respectively 893 μs and 66.44%, higher than those of Eu(III)-thenoyltrifluoroacetone-1,10-phenanthroline complex [Eu(TTA)3phen]. The result of TG-DSC measurement showed the enhanced thermal stability of Eu-HTlc compared with that of MgAl-LDHs and Eu(TTA)3phen. With excellent photoluminescent property and thermal stability, low contents of rare earth ions and ligands, the Eu-HTlc may become one of the novel fluorescent materials with potential applications.
Similar content being viewed by others
References
Canavi F, Trifiro F, Vaccari A. Hydrotalcite-type anionic clays: preparation, properties and applications. Catal Today, 1991, 11(2): 173–301
Vaccari A. Preparation and catalytic properties of cationic and anionic clays. Catal Today, 1998, 41: 53–71
Weitkamp J, Hunger M, Rymsa U. Base catalysis on microporous and mesoporous materials: recent progress and perspectives. Micro-porous Mesoporous Mater, 2001, 48: 255–270
Khan AI, O’Hare DJ. Intercalation chemistry of layered double hydroxides: recent developments and applications. Mater Chem, 2002, 12: 3191–3198
Sels BF, De Vos DE, Jacobs PA. Hydrotalcite-like anionic clays in catalytic organic reactions. Catal Rev, 2001, 43: 443–488
Costantino U, Ambrogi V, Nocchetti M, Perioli L. Hydrotalcite-like compounds: versatile layered hosts of molecular anions with biological activity. Micropor Mesopor Mater, 2008, 107(1–2): 149–160
Choy JH, Jung JS, Oh JM, Park M, Jeong J, Kang YK, Han OJ. Layered double hydroxide as an efficient drug reservoir for folate derivatives. Biomaterials, 2004, 25: 3059–3064
Choy JH., Choi SJ, Oh JM, Park T. Clay minerals and layered double hydroxides for novel biological applications. Appl Clay Sci, 2007, 36:122–132
Aisawa S, Sasaki S, Takahashi S, Hirahara H, Nakayama H, Narita E. Intercalation of amino acids and oligopeptides into Zn-Al layered double hydroxide by coprecipitation reaction. J Phys Chem Solids, 2006, 67: 920–925
Ambrogi V, Fardella G, Grandolini G, Perioli L. Intercalation compounds of hydrotalcite-like anionic clays with antiinflammatory agents-I. Intercalation and in vitro release ofibuprofen. Int J Pharm, 2001, 220: 23–32
Mignani A, Scavetta E, Tonelli D. Electrodeposited glucose oxidase/anionic clay for glucose biosensors design. Anal Chim Acta, 2006, 577:98–106
Mousty C, Vieille L, Cosnier S. Laccase immobilization in redox active layered double hydroxides: a reagentless amperometric biosensor. Biosensors Bioelectronics, 2007, 22(8): 1733–1738
Fudala Á, Pálinkó I, Kiricsi I. Preparation and characterization of hybrid organic-inorganic composite materials using the amphoteric property of amino acids: amino acid intercalated layered double hydroxide and montmorillonite. Inorg Chem, 1999, 38(21): 4653–4658
Tammaro L, Costantino U, Bolognese A, Sammartino G, Marenzi G, Calignano A, Tete S, Mastrangelo F, Califano L, Vittoria V. Nanohybrids for controlled antibiotic release in topical applications. Int J. Antimicrob Agents, 2007, 29(4): 417–423
Weissman SI. Intramolecular energy transfer the fluorescence of complexes of europium. J Chem Phys, 1942, 10: 214–217
Halverson F, Brinen JS, Leto JR. Photoluminescence of lanthanide complexes. III. synergic agent complexes involving extended chromophores. J Chem Phys, 1964, 41: 2752–2759
Ci YX, Lan ZH. Fluorescence enhancement of the europium(III)-thenoyltrifluoroacetone-trioctylphosphine oxide ternary complex by gadolinium(III) and its application to the determination of europium( III). Analyst, 1988, 113: 1453–1457
Li WL, Yu G, Zhao X. The preparation and luminescence of Eu(III) activated o-phenanthroline containing Gd(III) succinate phosphors. J Alloys Compd, 1994, 206: 195–199
Zhong GL, Yang KZ. Luminescence enhancement effect of Y(TTA)3Phen on Europium(III) and intermolecular energy transfer in Langmuir-Blodgett films. Langmuir, 1998, 14(19): 5502–5506
Wang LH, Wang W, Zhang WG, Kang ET, Huang W. Synthesis and luminescence properties of novel Eu-containing copolymers consisting of Eu(III)-acrylate-β-diketonate complex monomers and methyl methacrylate. Chem Mater, 2000, 12(8): 2212–2218
Piguet C, Buenzli JCG, Bernardinelli G, Hopfgartner G, Williams AF. Self-assembly and photophysical properties of lanthanide dinuclear triple-helical complexes. J Am Chem Soc, 1993, 115(18): 8197–8206
Qian DJ, Yang KZ, Nakahara H, Fukuda K. Monolayers of europium complexes with different long chains and β-diketonate ligands and their emission properties in Langmuir-Blodgett films. Langmuir, 1997, 13: 5925–5932
Campos RA, Kovalev IP, Guo Y, Wakili N, Skotheim T. Red electroluminescence from a thin organometallic layer of europium. J Appl Phys, 1996, 80: 7144–7150
Gao XC, Cao H, Huang CH, Li BG, Umitani S. Electroluminescence of a novel terbium complex. Appl Phys Lett, 1998, 72: 2217–2219
Tsutsui T, Takada N, Saito S, Ogino E. Sharply directed emission in organic electroluminescent diodes with an opticalmicrocavity structure. Appl Phys Lett, 1994, 65: 1868–1870
Stumpf T, Curtius H, Walther C, Dardenne K, Ufer K, Fanghaenel T. Incorporation of Eu(III) into Hydrotalcite: a TRLFS and EXAFS Study. Environ Sci Technol, 2007, 41(9): 3186–3191
Sandra G, Martyn P, Rute A, Sá F, Luís D, Carlos TM, Santos IS, Goncüalves. Immobilization of lanthanide ions in a pillared layered double hydroxide. Chem Mater, 2005, 17: 5803–5809
Liu JC, Lian Sh X, Zhu Al, Li QH, Liu LM, Zeng LH. Luminescent properties and selectivity IR adsorption of Eu-doped hydrotalcite like compounds. Chi J Lumin, 2007, 28(1): 67–73
Zhang WG, Chen H. Preparation and characterization of a novel strong-fluorescent hydrotalcite-like compound (Al-HTLc). Science in China Series B: Chem, 2008, 51(9): 834–841
Chen H, Zhang WG. Study on the strong-fluorescent Zn-containing hydrotalcite-like compound. Acta Chim Sinica, 2008, 66(4): 481–486
Miyata S. Anion-exchange properties of hydrotalcite-like compounds. Clays Clay Miner, 1983, 31: 305–311
Wang Ch Y, Yang ZJ, Li Y, Gong LW, Zhao GW. Preparation of thin films of ternary complex of Europium with 2-thenoyltrifluoroacetone and o-phenanthroline. Phys Stat Sol(a), 2002, 191(1): 117–124
Fatiha BDS, Su BL, Noureddine B. Removal of evans blue by using nickel-iron layered double hydroxide (LDH) nanoparticles: effect of hydrothermal treatment temperature on textural properties and dye adsorption. Macromol Symp, 2008, 273: 125–134
Gupta S, Agarwal DD, Banerjee S. Synergistic combination of metal stearates and β-diketones with hydrotalcites in poly(vinyl chloride) stabilization. J Appl Polym Sci, 2009, 112: 1056–1062
Hernandez-Moreno MJ, Ulibarri MA, Rendon JL, Serna CJ. IR characteristics of hydrotalcite-like compounds. Phys Chem Miner, 1985, 12: 34–38
Gou GJ, Ma PH, Chu MX. Dynamics of intercalation of B4O5 (OH) 2−4 anion into layered double hydroxides intercalated by Cl− anion. Chem J Chi Univ, 2005, 26(3): 497–502
Kagunya W, Baddor-Hadjean R, Kooli F, Jones W. Vibrational modes in layered double hydroxides and their calcined derivatives. Chem Phys, 1998, 236: 225–234
Chen SP, Meng XX, Shuai Q, Jiao BJ, Gao SL, Shi QZ. Thermochemistry of Europium and dithiocarbamate complex Eu(C5H8NS2)3−(C12H8N2). J Therm Analy Calori, 2006, 86(3): 767–774
Xiao LR, Gao F, Tang JY, Zhang WG. Study on the Eu-containing coordination polymer I. structural characterization of Eu (III)-thienylt rifluroacetone-poly(styrene-acrylic acid). Spectroscopy Spectral Analysis, 2004, 24(6): 756–761
Bellotto M, Rebours B, Clause O, Lynch J, Bazin D, Elkaim E A. A Reexamination of hydrotalcite crystal chemistry. J Phys Chem, 1996, 100(20): 8527–8534
Lee WF, Lee S Ch. Effect of hydrotalcite on the swelling and mechanical behaviors for the hybrid nanocomposite hydrogels based on gelatin and hydrotalcite. J Applied Polymer Sci, 2006, 100: 500–507
Zhang L, Shen YH, Xie AJ, Li Sh K, Jin BK, Zhang QF. One-step synthesis of monodisperse silver nanoparticles beneath Vitamin E langmuir monolayers. J Phys Chem B, 2006, 110: 6615–6620
Hu J, Zhao H, Zhang QJ, He WD. Synthesis and characterization of submicron PMMA particles containing rare earth ions on the surface. J Appl Polym Sci, 2003, 89: 1124–1131
Kirby AF, Richardson FS. Detailed analysis of the optical absorption and emission spectra of europium(3+) in the trigonal (C3) Eu(DBM)3.H2O system. J Phys Chem, 1983, 87: 2544–2556
Chen G Zh, Huang X Zh, Zheng Zh Zh. Fluorescence Analytical Method. 2nd ed. Beijing: Science Press, 1990. 15–17
de Mello BJC, Wittmann HF, Friend RH. An improved experimental determination of external photoluminescence quantum efficiency. Adv Mater, 1997, 9(3): 230–232
Newman SP, Jones W. Synthesis, characterization and applications of layered double hydroxides containing organic guests. New J Chem, 1998, 105–115
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, H., Zhang, W. Synthesis and characterization of a strong-fluorescent Eu-containing hydrotalcite-like compound. Sci. China Chem. 53, 1273–1280 (2010). https://doi.org/10.1007/s11426-010-3167-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-010-3167-0