Abstract
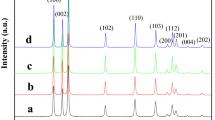
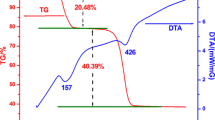
ZnO nanoparticles with different morphologies were solvothermally synthesized by controlling the alkali (sodium hydroxide) concentration in an isopropanol solution. The products were characterized by means of powder X-ray diffraction, UV-visible absorption spectra, scanning electron microscopy, transmission electron microscopy, and selected area electron diffraction. The morphologies of the formed ZnO nanocrystals were dependent on the concentration of the alkali, and with increases of sodium hydroxide concentration, the ZnO nanocrystals evolved from rod to hexagonal bipyramid, and then to a flower-like nanostructure. The flower-like nanostructure resulted from the etching of the hexagonal bipyramid by the excess alkali. The photoluminescence and photocatalytic properties of the prepared ZnO were investigated. The difference of green emission among the ZnO nanocrystals indicated that a higher sodium hydroxide concentration led to a higher level of defects. The size, the surface structure and defects in the ZnO nanocrystals affected its photo-degradation characteristics.
Similar content being viewed by others
References
Katoh R, Furube A, Hara K, Murata S, Sugihara H, Arakawa H, Tachiya M. Efficiences of electron injection from excited sensitizer dyes to nanocrystalline ZuO films as studied by near-IR optical absorption of injected electrons. J Phys Chem B, 2002, 106(50): 12957–12964
Dong LF, Cui ZL, Zhang ZK. Gas sensing properties of nano-ZnO pre pared by arc plasma method. Nanostruct Mater, 1997, 8(7): 815–823
Chadwick AV, Russell NV, Whitham AR, Wilson A. Nanocrystalline metal oxide gas sensors. Sensor Actuat B, 1994, 18(1–3): 99–102
Huang MH, Mao S, Feick H, Yan HQ, Wu YY, Kind H, Weber E, Russo R, Yang PD. Room-temperature ultraviolet nanowire nanolasers. Science, 2001, 292(5523): 1897–1899
Kong XY, Wang ZL. Spontaneous polarization-inducednanohelixes, nanosprings and nanorings of piezoelectric nanobelts. Nano Lett, 2003, 3(12): 1625–1631
Minne SC, Manalis SR, Quate CF. Parallel atomic force microscopy using cantilevers with integrated piezoresistive sensors and integrated piezoelectric actuators. Appl Phys Lett, 1995, 67(26): 3918–3920
Ressler T, Kniep BL, Kasatkin I, Schlög R. The microstructure of copper zinc oxide catalysts: bridging the materials gap. Angew Chem Int Ed, 2005, 44, 4704–4707
Service RF. Materials science—will UV lasers beat the blues. Science, 1997, 276(5314): 895–895
Guo L, Ji YL, Xu H. Regularly shaped, single-crystalline ZnO nanorodswith wurtzite structure. J Am Chem Soc, 2002, 124(50): 14864–14865
Yu HD, Zhang ZP, Han MY, Hao XT, Zhu FR. A general lowtemperature route for large-scale fabrication of highly oriented ZnO nanorod/nanotube arrays. J Am Chem Soc, 2005, 127(8): 2378–2379
Vayssieres L, Keis K, Hagfeldt A, Lindquist SE. Three-dimensional array of highly oriented crystalline ZnO Microtubes. Chem Mater, 2001, 13(12): 4395–4398
Sun Y, Fuge GM, Fox NA, Riley DJ, Ashfold MNR. Synthesis of aligned arrays of ultrathin ZnO nanotubes on a Si wafer coated with a thin ZnO film. Adv Mater, 2005, 17(20): 2477–2481
Chen SJ, Liu YC, Shao CL, Mu R, Lu YM, Zhang JY, Shen DZ, Fan XW. Structural and optical properties of uniform ZnO nanosheets. Adv Mater, 2005, 17(5): 586–590
Dai Y, Zhang Y, Li QK, Nan CW. Synthesis and optical properties of tetrapod-like zinc oxide nanorods. Chem Phys Lett, 2002, 358(1–2): 83–86
Tian N, Zhou ZY, Sun SG, Ding Y, Wang ZL. Synthesis of tetrahexahedral platinum nanocrystals with high-index facets and high electro-oxidation activity. Science, 2007, 316(5825): 732–735
Alivisatos AP. Semiconductor cluster, nanocrystals, and quantum dots. Science, 1996, 271(5251): 933–937
Yanagida T, Marcu A, Matsui H, Nagashima K, Oka K, Yakota K, Taniguchi M, Kawai T. Enhancement of oxide VLS growth by carbon on substrate surface. J Phys Chem C, 2008, 112(48): 18923–18926
Wu JJ, Wu SC. Low-temperature growth of well-aligned ZnO nanorods by chemical vapor deposition. Adv Mater, 2002, 14(3): 215–218
Guo Y, Wang H, He C, Qiu L, Cao X. Uniform carbon-coated ZnO nanorods: microwave-assisted preparation, cytotoxicity, and photocatalytic activity. Langmuir, 2009, 25(8): 4678–4684
Sounart TL, Liu J, Voigt JA, Hsu JWP, Spoerke ED, Tian ZR, Jiang YB. Sequential nucleation and growth of complex nanostructured films. Adv Funct Mater, 2006, 16(3): 335–344
Cozzoli PD, Kornowski A, Weller H. Colloidal synthesis of organic-capped ZnO nanocrystals via a sequential reduction-oxidation reaction. J Phys Chem B, 2005, 109(7): 2638–2644
Jun YW, Lee JH, Choi J, Cheon J. Symmetry-controlled colloidal nanocrystals: nonhydrolytic chemical synthesis and shape determining parameters. J Phys Chem B, 2005, 109(31): 14795–14806
Zhang J, Sun LD, Yin JL, Su HL, Liao CS, Yan CH. Control of ZnO morphology via a simple solution route. Chem Mater, 2002, 14(10): 4172–4177
Li ZK, Huang XT, Liu JP, Li YY, Li GY. Morphology control and transition of ZnO nanorod arrays by a simple hydrothermal method. Mater Lett, 2008, 62(10–11): 1503–1506
Bahnemann DW, Kormann C, Hoffmann MR. Preparation and characterization of quantum size zinc oxide: a detailed spectroscopic study. J Phys Chem, 1987, 91(14): 3789–3798
Yang HG, Liu G, Qiao SZ, Sun CH, Jin YG, Smith SC, Zou J, Cheng HM, Lu GQ. Solvothermal synthesis and photoreactivity of anatase TiO2 nanosheets with dominant {001} facets. J Am Chem Soc, 2009, 131(11): 4078–4083
Tandra G, Soumitra K, Jay G, Subhadra C. ZnO nanocones: solvothermal synthesis and photoluminescence properties. Mater Res Bull, 2008, 43(8–9): 2228–2238
Zhou X, Kuang Q, Jiang ZY, Xie ZX, Xu T, Huang RB, Zheng LS, The origin of green emission of ZnO microcrystallites: surfacedependent light emission studied by cathodoluminescence. J Phys Chem C, 2007, 111(32): 12091–12093
Li F, Ding Y, Gao PX, Xin XQ, Wang ZL. Single-crystal hexagonal disks and ringsof ZnO: low-temperature, Large-scale synthesis and growth mechanism. Angew Chem Int Ed, 2004, 43(39): 5238–5242
DjurišiĆ AB, Leung YH. Optical properties of ZnO nanostructures. Small, 2006, 2(8–9): 944–961
Jiang P, Zhou JJ, Fang HF, Wang CY, Wang ZL, Xie SS. Hierarchical shelled ZnO structures made of bunched nanowire arrays. Adv Funct Mater, 2007, 17(8): 1303–1310
Han XG, He HZ, Kuang Q, Zhou X, Zhang XH, Xu T, Xie ZX, Zheng LS. Controlling morphologies and tuning the related properties of nano/microstructured ZnO crystallites. J Phys Chem C, 2009, 113(2): 584–589
Zheng Y, Chen C, Zhan Y, Lin X, Zheng Q, Wei K, Zhu J, Zhu Y. Luminescence and photocatalytic activity of ZnO nanocrystals: Correlation between structure and property. Inorg Chem, 2007, 46(16): 6675–6682
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Jiang, Y., Li, F., Sun, R. et al. A simple solvothermal route towards the morphological control of ZnO and tuning of its optical and photocatalytic properties. Sci. China Chem. 53, 1711–1717 (2010). https://doi.org/10.1007/s11426-010-3160-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-010-3160-7