Abstract
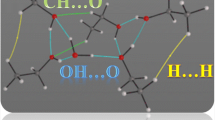
In separation processes, hydrogen bonding has a very significant effect on the efficiency of isolation of acetic acid (HOAc) from HOAc/H2O mixtures. This intermolecular interaction on aggregates composed of a single HOAc molecule and varying numbers of H2O molecules has been examined by using ab initio molecular dynamics simulations (AIMD) and quantum chemical calculations (QCC). Thermodynamic data in aqueous solution were obtained through the self-consistent reaction field calculations and the polarizable continuum model. The aggregation free energy of the aggregates in gas phase as well as in aqueous system shows that the 6-membered ring is the most favorable structure in both states. The relative stability of the ring structures inferred from the thermodynamic properties of the QCC is consistent with the ring distributions of the AIMD simulation. The study shows that in dilute aqueous solution of HOAc the more favorable molecular interaction is the hydrogen bonding between HOAc and H2O molecules, resulting in the separation of acetic acid from the HOAc/H2O mixtures with more difficulty than usual.
Similar content being viewed by others
References
Chang H C, Jiang J C, Lin M S, Kao H E, Feng C M, Huang Y C, Lin S H. On the search for C-H-O hydrogen bonding in aqueous acetic acid: Combined high-pressure infrared spectroscopy and ab initio calculations study. J Chem Phys, 2002, 117: 3799–3803
Karle J, Brockway L O. An electron-diffraction investigation of the monomers and dimers of formic, acetic and trifluoroacetic acids and the dimer of deuterium acetate. J Am Chem Soc, 1944, 66: 574–584
Frurip D J, Curtiss L A, Blander M. Vapor phase association in acetic and trifluoroacetic acids. Thermal conductivity measurements and molecular orbital calculations. J Am Chem Soc, 1980, 102: 2610–2616
Davis J C Jr, Pitzer K S. Nuclear magnetic resonance (NMR) studies of hydrogen bonding. I. Carboxylic acids. J Phys Chem, 1960, 64: 886–892
Waldstein P, Blatz L A. Low-frequency Raman spectra and molecular association in liquid formic and acetic acids. J Phys Chem, 1967, 71: 2271–2276
Tjahjono M, Allian A D, Garland M. Experimental dipole moments for nonisolatable acetic acid structures in a nonpolar medium. A combined spectroscopic, dielectric, and DFT study for self-association in solution. J Phys Chem B, 2008, 112: 6448–6459
Nakabayashi T, Kosugi K, Nishi N. Liquid structure of acetic acid studied by Raman spectroscopy and ab initio molecular orbital calculations. J Phys Chem A, 1999, 103: 8595–8603
Freedman E. The use of ultrasonic absorption for the determination of very rapid reaction rates at equilibrium: application to the liquid-phase association of carboxylic acids. J Chem Phys, 1952, 21: 1784–1790
Cartwright D R, Monk C B. The molecular association of some carboxylic acids in aqueous solutions from conductivity data. J Chem Soc, 1955, 2500-2503
Ng J B, Shurvell H F. A study of the self-association of acetic acid in aqueous solution using raman spectroscopy. Can J Spectrosc, 1985, 30: 149–153
Ng J B, Shurvell H F. Application of factor analysis and band contour resolution techniques to the Raman spectra of acetic acid in aqueous solution. J Phys Chem, 1987, 91: 496–500
Tanaka N, Kitano H, Ise N. Raman spectroscopic study of hydrogen bonding in aqueous carboxylic acid solutions. J Phys Chem, 1990, 94: 6290–6292
Johnson C M, Tyrode E, Baldelli S, Rutland M W, Leygraf C. A vibrational sum frequency spectroscopy study of the liquid-gas interface of acetic acid-water mixtures: 1. Surface speciation. J Phys Chem B, 2005, 109: 321–328
Tyrode E, Johnson C M, Baldelli S, Leygraf C, Rutland M W. A vibrational sum frequency spectroscopy study of the liquid-gas interface of acetic acid-water mixtures: 2. Orientation analysis. J Phys Chem B, 2005, 109: 329–341
Nishi N, Nakabayashi T, Kosugi K. Raman spectroscopic study on acetic acid clusters in aqueous solutions: Dominance of acid-acid association producing microphases. J Phys Chem A, 1999, 103: 10851–10858
Colominas C, Teixido J, Cemeli J, Luque F J, Orozco M. Dimerization of carboxylic acids: Reliability of theoretical calculations and the effect of solvent. J Phys Chem B, 1998, 102: 2269–2276
Aquino A J A, Tunega D, Haberhauer G, Gerzabek M H, Lischka H. Solvent effects on hydrogen bonds-a theoretical study. J Phys Chem A, 2002, 106: 1862–1871
Chocholoušová J, Vacek J, Hobza P. Acetic acid dimer in the gas phase, nonpolar solvent, microhydrated environment, and dilute and concentrated acetic acid: Ab initio quantum chemical and molecular dynamics simulations. J Phys Chem A, 2003, 107: 3086–3092
Génin F, Quilès F, Burneau A. Infrared and Raman spectroscopic study of carboxylic acids in heavy water. PCCP, 2001, 3: 932–942
Takamuku T, Kyoshoin Y, Noguchi H, Kusano S, Yamaguchi T. Liquid structure of acetic acid-water and trifluoroacetic acid-water mixtures studied by large-angle X-ray scattering and NMR. J Phys Chem B, 2007, 111: 9270–9280
Ouyang B, Howard B J. The monohydrate and dihydrate of acetic acid: A high-resolution microwave spectroscopic study. PCCP, 2009, 11: 366–373
Pu L, Wang Q, Zhang Y, Miao Q, Kim Y S, Zhang Z B. Architecture of hydrates and local structure of acetic acid aqueous solution: Ab initio calculations and Car-Parrinello molecular dynamics (CPMD) simulations on hydrogen-bonding rings, network, and intra-hydrate protonation in multi-hydrates of acetic acid monomer. Adv Quantum Chem, 2008, 54: 271–295
Pu L, Sun Y M, Zhang Z B. Hydrogen bonding of hydrates of double acetic acid molecules. J Phys Chem A, 2009, 113: 6841–6848
Car R, Parrinello M. Unified approach for molecular dynamics and density-functional theory. Phys Rev Lett, 1985, 55: 2471–2474
CPMD, Copyright IBM Corp 1990–2004, Copyright MPI für Festkörperforschung Stuttgart 1997–2001
Becke A D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A, 1988, 38: 3098–3100
Lee C, Yang W, Parr R G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B, 1988, 37: 785–789
Zhan C G, Dixon D A. Absolute hydration free energy of the proton from first-principles electronic structure calculations. J Phys Chem A, 2001, 105: 11534–11540
Zhan C G, Dixon D A. Hydration of the fluoride anion: Structures and absolute hydration free energy from first-principles electronic structure calculations. J Phys Chem A, 2004, 108: 2020–2029
Cancès M T, Mennucci B, Tomasi J, A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to isotropic and anisotropic dielectrics. J Chem Phys, 1997, 107: 3032–3041
Cossi M, Barone V, Mennucci B, Tomasi J. Ab initio study of ionic solutions by a polarizable continuum dielectric model. Chem Phys Lett, 1998, 286: 253–260
Mennucci B, Tomasi J, Continuum solvation models: A new approach to the problem of solute’s charge distribution and cavity boundaries. J Chem Phys, 1997, 106: 5151–5158
Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Zakrzewski V G, Montgomery Jr J A, Stratmann R E, Burant J C, Dapprich S, Millam J M, Daniels A D, Kudin K N, Strain M C, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson G A, Ayala P Y, Cui Q, Morokuma K, Malick D K, Rabuck A D, Raghavachari K, Foresman J B, Cioslowski J, Ortiz J V, Stefanov B B, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin R L, Fox D J, Keith T, Al-Laham M A, Peng C Y, Nanayakkara A, Gonzalez C, Challacombe M, Gill P M W, Johnson B, Chen W, Wong M W, Andres J L, Gonzalez C, Head-Gordon M, Replogle E S, Pople J A. Gaussian 98. Revision A.6. Gaussian Inc. Pittsburgh PA. 1998
Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Montgomery Jr J A, Vreven T, Kudin K N, Burant J C, Millam J M, Iyengar S S, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson G A, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox J E, Hratchian H P, Cross J B, Adamo C, Jaramillo J, Gomperts R, Stratmann R E, Yazyev O, Austin A J, Cammi R, Pomelli C, Ochterski J W, Ayala P Y, Morokuma K, Voth G A, Salvador P, Dannenberg J J, Zakrzewski V G, Dapprich S, Daniels A D, Strain M C, Farkas O, Malick D K, Rabuck A D, Raghavachari K, Foresman J B, Ortiz J V, Cui Q, Baboul A G, Clifford S, Cioslowski J, Stefanov B B, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin R L, Fox D J, Keith T, Al-Laham M A, Peng C Y, Nanayakkara A, Challacombe M, Gill P M W, Johnson B, Chen W, Wong M W, Gonzalez C, Pople J A. Gaussian 03. Revision C.02. Gaussian Inc. Wallingford CT. 2004
Boys S F, Bernardi F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys, 1970, 19: 553–566
Gao Q, Leung K T. Hydrogen-bonding interactions in acetic acid monohydrates and dihydrates by density-functional theory calculations. J Chem Phys, 2005, 123: 074325
Pu L, Miao Q, Xu H L, Zhang L L, Zhang Z B. Perception of hydrogen bonding ring in a local structure of aqueous solution in the CPMD simulation (in Chinese). Comput Appl Chem, 2007, 24: 1324–1328
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supported by the Jiangsu Planned Projects for Postdoctoral Research Funds (No. 0901001C), the National Natural Science Foundation of China (Grant No. 20876072) and the Natural Science Foundation of Jiangsu Province (No. KB2008023)
Rights and permissions
About this article
Cite this article
Pu, L., Sun, Y. & Zhang, Z. Hydrogen bonding of single acetic acid with water molecules in dilute aqueous solutions. Sci. China Ser. B-Chem. 52, 2219–2225 (2009). https://doi.org/10.1007/s11426-009-0288-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-009-0288-4