Abstract
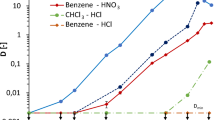
The extraction of strontium ions using DCH18C6 as the extractant and various ionic liquids (ILs) as solvents has been investigated. The distribution ratio of Sr2+ can reach as high as 103 under certain conditions, much larger than that in DCH18C6/n-octanol system. The extraction capacity depends greatly on the structure of ionic liquids. In IIs-based extraction systems, the extraction efficiency of strontium ions is reduced by increasing the concentration of nitric acid and can also be influenced directly by the presence of Na+ and K+ in the aqueous phase. It is confirmed that the extraction proceeds mainly via a cation-exchange mechanism.
Similar content being viewed by others
Reference
He L H, Weng X M, Yang D Z, Jiao R Z, Song C L. Removal of strontium from high-level radioactive waste by crown-ether extraction. Chin J Nucl Sci Eng (in Chinese), 1995, 15(3): 259–263
Yang Q, Han Y D, Liu D M. Extraction of Sr2+, Cs+ in simulated HLLW with crown ethers. J Nucl Radiochem (in Chinese), 1996, 18(01): 61–67
Ye G A, Luo F X, Jiang Y Q, Ding S D, Chen W J. Study on the extraction of strontium with amido podand. Atomic Energ Sci Tech (in Chinese), 2001, 35(04): 344–350
Dozol J F, Dozol M, Macias R M. Extraction of strontium and cesium by dicarbollides, crown ethers and functionalized calixarenes. J Incl Phenom Macrocycl Chem, 2000, 38: 1–22
Shen X H, Xu C, Liu X Q, Chu T W. Ionic liquids used in extraction and separation of metal ions. J Nucl Radiochem (in Chinese), 2006, 28(03): 129–138
Li R X. Green Solvents-Ionic liquids: Syntheses and Application (in Chinese). Beijing: Chemical Industry Press, 2004. 20–27
Rogers R D, Seddon K R. Ionic liquids-solvents of the future. Science, 2003, 302(5646): 792–793
Dai S, Ju Y H, Barnes C E. Solvent extraction of strontium nitrate by a crown ether using room-temperature ionic liquids. J Chem Soc, Dalton Trans, 1999, 8: 1201–1202
Visser A E, Swatloski R P, Reichert W M, Griffin S T, Rogers R D. Traditional extractants in nontraditional solvents: Groups 1 and 2 extraction by crown ethers in room-temperature ionic liquids. Ind Eng Chem Res, 2000, 39(10): 3596–3604
Dietz M L, Dzielawa J A. Ion-exchange as a mode of cation transfer into room-temperature ionic liquids containing crown ethers: Implications for the “Greenness” of ionic liquids as diluents in liquid-liquid extraction. Chem Commun, 2001, 20: 2124–2125
Yuan L Y, Peng J, Xu L, Zhai M L, Li J Q, Wei G S. Influence of gamma-radiation on the ionic liquid [C4mim][PF6] during extraction of strontium ions. J Chem Soc, Dalton Trans, 2008, 45, 6358–6360
Yuan L Y, Peng J, Xu L, Zhai M L, Li J Q, Wei G S. Radiation effect on hydrophobic ionic liquids [C4mim][NTf2] during extraction of strontium ions. J Phys Chem B, 2009, 113(26): 8948–8952
Bonhote P, Dias A P, Papageorgiou N, Kalyanasundaram K, Gratzel M. Hydrophobic, highly conductive ambient-temperature molten salts. Inorg Chem, 1996, 35(5): 1168–1178
Huddleston J G, Visser A E, Reichert W M, Willauer H D, Broker G A, Rogers R D. Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation. Green Chem, 2001, 3(4): 156–164
Huddleston J G, Willauer H D, Swatloski R P, Visser A E, Rogers R D. Room temperature ionic liquids as novel media for “clean” liquid-liquid extraction. Chem Commun, 1998(16): 1765–1766
Horwitz E P, Dietz M L, Fisher D E. Extraction of strontium from nitric-acid solutions using dicyclohexano-18-crown-6 and its derivatives. Solvent Extr Ion Exch, 1990, 8(4–5): 557–572
Dietz M L, Dzielawa J A, Laszak I, Young B A, Jensen M P. Influence of solvent structural variations on the mechanism of facilitated ion transfer into room-temperature ionic liquids. Green Chem, 2003, 5: 682–685
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China (Grant No. 20871009).
Rights and permissions
About this article
Cite this article
Xu, C., Shen, X., Chen, Q. et al. Investigation on the extraction of strontium ions from aqueous phase using crown ether-ionic liquid systems. Sci. China Ser. B-Chem. 52, 1858–1864 (2009). https://doi.org/10.1007/s11426-009-0268-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-009-0268-8