Abstract
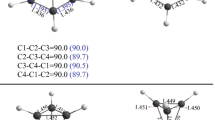
The reaction mechanism of CN radicals with ClO radicals has been studied theoretically using ab initio and density functional theory (DFT). The result shows that the main reaction path is the O atom in radical ClO attacks the C atom in radical CN to compose the intermediate 1 ClOCN. Three thermodynamically accessible prodncts, P1 (CO+ClN), P3 (NO+CCl), and P4 (ClNCO), were obtained from intermediate 1 through isomerization and decomposition reactions. P4 is the primary product, and P1 and P3 are the secondary product. Compared with the singlet potential energy surface, the contribution of the triplet potential energy surface can be ignored.
Similar content being viewed by others
References
Rowland F S. Stratospheric ozone depletion. Philos T Roy Soc B, 2006, 361: 769–790
Lee C, Kim Y J, Tanimoto H, Bobrowski N, Platt U, Mori T, Yamamoto K, Hong C S. High ClO and ozone depletion observed in the plume of Sakurajima volcano, Japan. Geophys Res Lett 2005, 32: L21809
Zhu R S, Lin M C. Ab initio studies of ClOx reactions: Prediction of the rate constants of ClO+NO2 for the forward and reverse processes. Chemphyschem 2005, 6: 1514–1521
Liu Y, Wang W L, Wang W N, Su K H, Zhang Y. Theoretical study on the multi-channel reaction of CH3S with ClO. J Mol Struc-Theochem, 2008, 866: 46–51
Zhu R S, Lin M C. Ab initio study of the ClO+NH2 reaction: Prediction of the total rate constant and product branching ratios. J Phys Chem A, 2007, 111: 3977–3983
Lary D J. Atomospheric pseudohalogen chemistry. Atmos Chem phys, 2004, 4: 5381–5405
Zhang W C, Du B N, Feng C J. Theoretical investigation of the reaction of CN with OCS. J Mol Struc-Theochem, 2005, 626: 25–30
Zhang W C, Du B N. Ab initio quantum chemical studies of reaction mechanism for CN with CH2CO. Int J Quantum Chem, 2006, 106: 1066–1085
Pang J L, Xie H B, Zhang S W, Ding Y H, Tang A Q. Theoretical study on reaction mechanism of fulminic acid HCNO with CN radical. J Phys Chem A, 2008 112: 5251–5256
Becke A D. Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys, 1993, 98(6): 5648–5652
Stephens P J, Devlin F J, Chabalowski C F, Frisch M J. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J Phys Chem, 1994, 98(45): 11623–11626
McLean A D, Chandler G S. Contracted Gaussian basis sets for molecular calculations. Second row atoms. J Chem Phys, 1980, 62(10): 5639–5648
Krishnan R, Binkley J S, Seeger R, Pople J A. Self-consistent molecular orbital methods. A basis set for correlated wave functions. J Chem Phys, 1980, 62(1): 650–654
Frisch M J, Pople J A, Binkley J S. Self-consistent molecular orbital methods 25: Supplementary functions for Gaussian basis sets. J Chem Phys, 1984, 80(6): 3265–3269
Gonzalez C, Schlegel H B. An improved algorithm for reaction path following. J Chem Phys, 1989, 90(4): 2154–2159
Gonzalez C, Schlegel H B. Reaction path following in mass-weighted internal coordinates. J Phys Chem, 1990, 94(14): 5523–5526
Kurita N, Kobayashi K, Kumahora H, Tago K, Ozawa K. Chem Phys Lett, 1992, 198: 95
Pople J A, Head-Gordon M, Raghavachari K. Quadratic configuration interaction. A general technique for determining electron correlation energies. J Chem Phys, 1986, 86(10): 5968–5965
Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Zakrzewski V G, Montgomery J A Jr, Stratmann R E, Burant J C, Dapprich S, Millam J M, Daniels A D, Kudin K N, Strain M C, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson G A, Ayala P Y, Cui Q, Morokuma K, Rega N, Salvador P, Dannenberg J J, Malick D K, Rabuck A D, Raghavachari K, Foresman J B, Cioslowski J, Ortiz J V, Baboul A G, Stefanov B B, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin R L, Fox D J, Keith T, Al-Laham M A, Peng C Y, Nanayakkara A, Challacombe M, Gill P M W, Johnson B, Chen W, Wong M W, Andres J L, Gonzalez C, Head-Gordon M, Replogle E S, Pople J A. 6Gaussian 98 (Revision A.11.2), Pittsburgh: Gaussian Inc, 2001
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China (Grant No. 20663048)
Rights and permissions
About this article
Cite this article
Yang, Y., Huang, X. & Sun, C. Theoretical study on the reaction of CN radicals with ClO radicals by density functional theory. Sci. China Ser. B-Chem. 52, 1973–1979 (2009). https://doi.org/10.1007/s11426-009-0256-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-009-0256-z