Abstract
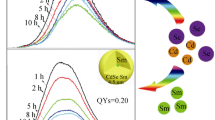
CdS:Mn nanorods have been produced via a solvothermal approach in the nonaqueous solvent of ethylenediamine. An absolutely dominant single Mn2+ emission originating from the d-d (4T1-6A1) transition was obtained in CdS:Mn nanocrystals at room temperature. The effects of varying reaction temperature, molar ratio of S/Cd, and reaction time on the crystallinity and luminescence of CdS:Mn nanocrystals were systematically investigated. 1% Mn2+-doped CdS nanorods without any other additives were synthesized at 130°C for 10 h with an S/Cd molar ratio of 2:1. They show a rod-like shape, and their luminescence intensity around 593 nm is almost the strongest of all the nanorod samples investigated. CdS:Mn nanorods promise potential applications in nanoscale electronic and photonic devices.
Similar content being viewed by others
References
Yang J, Zeng J H, Yu S H, Yang L, Zhou G E, Qian Y T. Formation process of CdS nanorods via solvothermal route. Chem Mater, 2000, 12: 3259–3263
Chu H B, Li X M, Chen G D, Zhou W W, Zhang Y, Jin Z, Xu J J, Li Y. Shape-controlled synthesis of CdS nanocrystals in mixed solvents. Cryst Growth Des, 2005, 5: 1801–1806
Zhang P, Gao L. Synthesis and characterization of CdS nanorods via hydrothermal microemulsion. Langmuir, 2003, 19: 208–210
Li Y D, Liao H W, Ding Y, Qian Y T, Yang L, Zhou G E. Nonaqueous synthesis of CdS nanorod semiconductor. Chem Mater, 1998, 10: 2301–2303
Na C W, Han D S, Kim D S, Kang Y J, Lee J Y, Park J. Photoluminescence of Cd1−x MnxS (x⩽0.3) nanowires. J Phys Chem B, 2006, 110: 6699–6704
Yong K T, Sahoo Y, Swihart M T, Prasad P N. Shape control of CdS nanocrystals in one-pot synthesis. J Phys Chem C, 2007, 111: 2447–2458
Chen X J, Xu H F, Xu N S, Zhao F H, Lin W J, Lin G, Fu Y L, Huang Z L, Wang H Z, Wu M M. Kinetically controlled synthesis of wurtzite ZnS nanorods through mild thermolysis of a covalent organic-inorganic network. Inorg Chem, 2003, 42: 3100–3106
Ye C H, Meng G W, Wang Y H, Jiang Z, Zhang L D. On the growth of CdS nanowires by the evaporation of CdS nanopowders. J Phys Chem B, 2002, 106: 10338–10341
Jun Y W, Lee S M, Kang N J, Cheon J. Controlled synthesis of multi-armed CdS nanorod architectures using monosurfactant system. J Am Chem Soc, 2001, 123: 5150–5151
Zhai T Y, Gu Z J, Zhong H Z, Dong Y, Ma Y, Fu H B, Li Y F, Yao J N. Design and fabrication of rocketlike tetrapodal CdS nanorods by seed-epitaxial metal-organic chemical vapor deposition. Cryst Growth Des, 2007, 7: 488–491
Dong L F, Gushtyuk T, Jiao J. Synthesis, characterization, and growth mechanism of self-assembled dendritic CdS nanorods. J Phys Chem B, 2004, 108: 1617–1620
Yao W T, Yu S H, Liu S J, Chen J P, Liu X M, Li F Q. Architectural control syntheses of CdS and CdSe nanoflowers, branched nanowires, and nanotrees via a solvothermal approach in a mixed solution and their photocatalytic property. J Phys Chem B, 2006, 110: 11704–11710
Nag A, Sapra S, Nagamani C, Sharma A, Pradhan N, Bhat S V, Sarma D D. A study of Mn2+ doping in CdS nanocrystals. Chem Mater, 2007, 19: 3252–3259
Bol A A, Meijerink A. Luminescence quantum efficiency of nanocrystalline ZnS:Mn2+. 1. Surface passivation and Mn2+ concentration. J Phys Chem B, 2001, 105: 10197–10202
Bol A A, Meijerink A. Luminescence quantum efficiency of nanocrystalline ZnS:Mn2+. 2. Enhancement by UV irradiation. J Phys Chem B, 2001, 105: 10203–10209
Denzler D, Olschewski M, Sattler K. Luminescence studies of localized gap states in colloidal ZnS nanocrystals. J Appl Phys, 1998, 84: 2841–2845
Dunstan D E, Hagfeldt A, Almgren M, Siegbahn H O G, Mukhtar E. Importance of surface reactions in the photochemistry of ZnS colloids. J Phys Chem, 1990, 94: 6797–6804
Sun J Q, Hao E C, Sun Y P, Zhang X, Yang B, Zou S, Shen J C, Wang S B. Multilayer assemblies of colloidal ZnS doped with silver and polyelectrolytes based on electrostatic interaction. Thin Solid Films, 1998, 327–329: 528–531
Luo X X, Cao W H, Zhou L X. Synthesis and luminescence properties of (Zn, Cd)S:Ag nanocrystals by hydrothermal method. J Lumin, 2007, 122–123: 812–815
Tata M, Banerjee S, John V T, Waguespack Y, Mcpherson G L. Fluorescence quenching of CdS nanocrystallites in AOT water-in-oil microemulsions. Colloid Surface A, 1997, 127: 39–46
Yang P, Song C F, Lv M K, Zhou G J, Yang Z X, Xu D, Yuan D R. Photoluminescence of Cu+-doped and Cu2+-doped ZnS nanocrystallites. J Phys Chem Solids, 2002, 63: 639–643
Gan L M, Liu B, Chew C H, Xu S J, Chua S J, Loy G L, Xu G Q. Enhanced photoluminescence and characterization of Mn-doped ZnS nanocrystallites synthesized in microemulsion. Langmuir, 1997, 13: 6427–6431
Zimnitsky D, Jiang C Y, Xu J, Lin Z Q, Tsukruk V V. Substrate- and time-dependent photoluminescence of quantum dots inside the ultrathin polymer LbL film. Langmuir, 2007, 23: 4509–4515
Bryant G W, Jaskolski W. Surface effects on capped and uncapped nanocrystals. J Phys Chem B, 2005, 109: 19650–19656
Wakefield G, Keron H A, Dobson P J, Hutchison J L. Structural and optical properties of terbium oxide nanoparticles. J Phys Chem Solids, 1999, 60: 503–508
Yu Z G, Pryor C E, Lau W H, Berding M A, MacQueen D B. Core-shell nanorods for efficient photoelectrochemical hydrogen production. J Phys Chem B, 2005, 109: 22913–22919
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China (Grant No. 50672089) and the Program for New Century Excellent Talents in University (NCET-08-0511)
Rights and permissions
About this article
Cite this article
Cao, L., Qu, H., Sun, D. et al. Solvothermal synthesis and luminescence properties of CdS:Mn nanorods. Sci. China Ser. B-Chem. 52, 2134–2140 (2009). https://doi.org/10.1007/s11426-009-0170-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-009-0170-4