Abstract
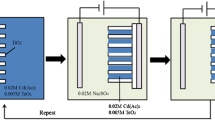
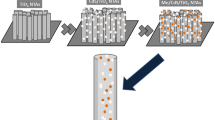
A constant current electrochemical deposition was employed to incorporate CdS nanoparticles into the TiO2 nanotube arrays (TiO2NTs). The size and amount of CdS nanoparticles in TiO2NTs (CdS@TiO2NTs) were controllable via modulating current, deposition time and electrolyte concentration. It was revealed, from the scanning electron microscopy (SEM) images and X-ray photoelectron spectroscopy (XPS) in depth profile, that CdS nanoparticles were filled into TiO2 nanotubes. A shift of the absorption edge toward the visible region under the optimal electrodeposition condition was observed with the diffuse reflectance spectroscopy (DRS). A 5-fold enhancement in the photocurrent spectrum for TiO2NTs was observed and the photocurrent response range was significantly extended into the visible region because of the CdS incorporation. Compared with pure TiO2NTs, under a visible light irradiation, CdS@TiO2NTs exhibited a 3.5-fold improvement of photocatalytic activity, which was demonstrated by the photocatalytic decomposition of Rhodamine B (RhB).
Similar content being viewed by others
References
Fujishima A, Rao T N, Trky D A. Titanium dioxide photocatalysis. J Photochem Photobiol C, 2000, 1: 1–21
Fujishima A, Zhang X. Titanium dioxide photocatalysis: Present situation and future approaches. C R Chim, 2006, 9: 750–760
Gong D, Grimes C A, Varghese O K, Hu W, Singh R S, Chen Z, Dickey E C. Titanium oxide nanotube arrays prepared by anodic oxidation. J Mater Res, 2001, 16: 3331–3334
Macák J M, Tsuchiya H, Schmuki P. High-aspect-ratio TiO2 nanotubes by anodization of titanium. Angew Chem Int Ed, 2005, 44: 2100–2102
Grimes C A. Synthesis and application of highly ordered arrays of TiO2 nanotubes. J Mater Chem, 2007, 17: 1451–1457
Zhuang H F, Lin C J, Lai Y K, Sun L, Li J. Some critical structure factors of titanium oxiden anotube array in its photocatalytic activity. Environ Sci Technol, 2007, 41: 4735–4740
Macák J M, Schmuki P. Anodic growth of self-organized anodic TiO2 nanotubes in viscous electrolytes. Electrochim Acta, 2006, 52: 1258–1264
Prakasam H E, Shankar K, Paulose M, Varghese O K, Grimes C A. A new benchmark for TiO2 nanotube array growth by anodization. J Phys Chem C, 2007, 111: 7235–7241
Kaneco S, Chen Y S, Westerhoff P, Crittenden J C. Fabrication of uniform size titanium oxide nanotubes: Impact of current density and solution conditions. Scripta Mater, 2007, 56: 373–376
Quan X, Yang S G, Ruan X L, Zhao H M. Preparation of titania nanotubes and their environmental applications as electrode. Environ Sci Technol, 2005, 39: 3770–3775
Macák J M, Zlamal M, Krysa J, Schmuki P. Self-organized TiO2 nanotube layers as highly efficient photocatalysts. Small, 2007, 3(2): 300–304
Varghese O K, Grimes C A. Appropriate strategies for determining the photoconversion efficiency of water photoelectrolysis cells: A review with examples using titania nanotube array photoanodes. Sol Energy Mater Sol Cells, 2008, 92: 374–384
Lai Y K, Sun L, Chen Y C, Zhuang H F, Lin C J, Chin J W. Effects of the structure of TiO2 nanotube array on Ti substrate on its photocatalytic activity. J Electrochem Soc, 2006, 153: D123–D127
Chen Y S, Crittenden J C, Hackney S, Sutter L, Hand D W. Preparation of a novel TiO2-based p-n junction nanotube photocatalyst. Environ Sci Technol, 2005, 39: 1201–1208
Xie Y B, Zhou L M, Huang H T. Enhanced photoelectrochemical current response of titania nanotube array. Mater Lett, 2006, 60: 3558–3560
Ghicov A, Macák J M, Tsuchiya H, Kunze J, Haeublein V, Frey L, Schmuki P. Ion implantation and annealing for an efficient N-doping of TiO2 nanotubes. Nano Lett, 2006, 6(5): 1080–1082
Paramasivam I, Macák J M, Ghicov A, Schmuki P. Enhanced photochromism of Ag loaded self-organized TiO2 nanotube layers. Chem Phys Lett, 2007, 445: 233–237
Ong K G, Varghese O K, Mor G K, Shankar K, Grimes C A. Application of finite-difference time domain to dye-sensitized solar cells: The effect of nanotube-array negative electrode dimensions on light absorption. Sol Energy Mater Sol Cells, 2007, 91: 250–257
Kongkanand A, Tvrdy K, Takechi K, Kuno M, Kamat P V. Quantum dot solar cells. Tuning photoresponse through size and shape control of CdSe-TiO2 architecture. J Am Chem Soc, 2008, 130(12): 4007–4015
Yin Y X, Jin Z G, Hou F. Enhanced solar water-splitting efficiency using core/sheath heterostructure CdS/TiO2 nanotube arrays. Nanotechnology, 2007, 18: No. 495608
Xiao M W, Wang L S, Wu Y D, Huang X J, Dang Z. Preparation and characterization of CdS nanoparticles decorated into titanate nanotubes and their photocatalytic properties. Nanotechnology, 2008, 19: No. 015706
Chen S G, Paulose M, Ruan C, Mor, G K, Varghese O K, Kouzoudis D, Grimes C A. Electrochemically synthesized CdS nanoparticle-modified TiO2 nanotube-array photoelectrodes: preparation, characterization, and application to photoelectrochemical cells. J Photochem Photobiol A, 2006, 177: 177–184
Sun W T, Yu Y, Pan H Y, Gao X F, Chen Q, Peng L M. CdS quantum dots sensitized TiO2 nanotube-array photoelectrodes. J Am Chem Soc, 2008, 130(4): 1124–1125
Xu D S, Xu Y J, Chen D P, Guo G L, Gui L L, Tang Y Q. Preparation and characterization of CdS nanowire arrays by dc electrodeposit in porous anodic aluminum oxide templates. Chem Phys Lett, 2000, 325: 340–344
Peng T Y, Yang H P, Dai K, Pu X L, Hirao K. Fabrication and characterization of CdS nanotube arrays in porous anodic aluminum oxide templates. Chem Phys Lett, 2003, 379: 432–436
Routkevitch D, Bigioni T, Moskovits M, Xu J M. Electrochemical fabrication of CdS nanowire arrays in porous anodic aluminum oxide templates. J Phys Chem, 1996, 100: 14037–14047
Barka N, Qourzal S, Assabbane A, Nounahb A, Ait-lchou Y. Factors influencing the photocatalytic degradation of Rhodamine B by TiO2-coated non-woven paper. J Photochem Photobio A, 2008, 195: 346–351
Kundu M, Khosravi A A, Kulkarni S K, Singh P. Synthesis and study of organically capped ultra small clusters of cadmium sulphite. J Mater Sci, 1997, 32: 245–258
Bandyopadhyay K, Mayya K S, Vijayamohanan K, Sastry M. Spontaneously organized molecular assembly of an aromatic organic disulfide on silver/platinum alloy surfaces: An angle dependent X-ray photoemission investigation. J Electron Spectrosc Relat Phenom, 1997, 87: 101–107
Ristova M, Ristov M. XPS profile analysis on CdS thin film modified with Ag by an ion exchange. Appl Surf Sci, 2001, 181: 68–77
Kokai J, Rakhshani A E. Photocurrent spectroscopy of solution grown CdS films annealed in CdCl2 vapour. J Phys D Appl Phys, 2004, 37: 1970–1975
Hidaka H, Nagaoka H, Nohara K, Shimura T, Horikoshi S, Zhao J, Serpone N. A mechanistic study of the photoelectrochemical oxidation of organic compounds on a TiO2/TCO particulate film electrode assembly. J Photochem Photobiol A, 1996, 98: 73–78
Hoffmann M R, Martin S T, Choi W, Bahnemann D W. Environmental applications of semiconductor photocatalysis. Chem Rev, 1995, 95: 69–96
Wu T X, Liu G M, Zhao J C. Photoassisted degradation of dye pollutants. V. Self-photosensitized oxidative transformation of Rhodamine B under visible light irradiation in aqueous TiO2 dispersions. J Phys Chem B, 1998, 102: 5845–5851
Bauer C, Jacques P, Kalt A. Investigation of the interaction between a sulfonated azo dye (AO7) and a TiO2 surface. Chem Phys Lett, 1999, 307: 397–406
Sun Z Y, Du J H, Chen H S, Gong W Q. FTIR study of nano-iron oxyhydroxides’ decolorationonthe azo dye. Spectrosc Spectral Anal, 2006, 26: 1226–1229
Li J Y, Ma W H, Lei P X, Zhao J C. Detection of intermediates in the TiO2-assisted photodegradation of Rhodamine B under visible light irradiation. J Environ Sci, 2007, 19: 892–896
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supported by the National Natural Science Foundation of China (Grant No. 50571085) and the Natural Science Foundation of Fujian Province (Grant No. U0750015) and R&D Program of Fujian (Grant No. 2007H0031) and that of Xiamen (Grant No. 3502Z20073004)
Rights and permissions
About this article
Cite this article
Wang, C., Sun, L., Xie, K. et al. Controllable incorporation of CdS nanoparticles into TiO2 nanotubes for highly enhancing the photocatalytic response to visible light. Sci. China Ser. B-Chem. 52, 2148–2155 (2009). https://doi.org/10.1007/s11426-009-0157-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-009-0157-1