Abstract
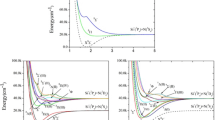
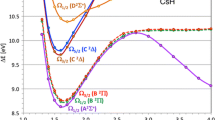
The potential energy surface crossings for 1,2-dithiete have been investigated using the complete active space self-consistent field (CASSCF) method and simple group theory. Using the full Pauli-Breit spin-orbit coupling (SOC) operator \( (\overset{\lower0.5em\hbox{$\smash{\scriptscriptstyle\frown}$}}{H} _{SO} ) \) which consists of the one-electron \( (\overset{\lower0.5em\hbox{$\smash{\scriptscriptstyle\frown}$}}{H} _{SO1} ) \) and two-electron \( (\overset{\lower0.5em\hbox{$\smash{\scriptscriptstyle\frown}$}}{H} _{SO2} ) \) terms, we estimate the strengths of the SOC (198.37 cm−1 when symmetry is imposed, and 211.35 cm−1 with no symmetry constraints), which plays an essential role in the spin transitions between different spin states. The calculations show that the photolysis of 1,3-dithiol-2-one leads to the formation of trans-dithioglyoxal (trans-MinS0) as a primary product which subsequently gives a secondary product identified as thiolthioketene. Our calculated results are in close agreement with the experimental observations.
Similar content being viewed by others
References
Celani P, Robb M A, Garavelli M, Bernardi F, Olivucci M. Geometry optimisation on a hypersphere. Application to finding reaction paths from a conical intersection. Chem Phys Lett, 1995, 243: 1–8
Reguero M, Olivucci M, Bernandi F, Robb M A. Excited-state potential surface crossings in acrolein: A model for understanding the photochemistry and photophysics of α,β-enones. J Am Chem Soc, 1994, 116: 2103–2114
Celani P, Ottani S, Olivucci M, Bernardi F, Robb M A. What happens during the picosecond lifetime of 2A1 cyclohexa-1,3-diene? A CAS-SCF study of the cyclohexadiene/hexatriene photochemical interconversion. J Am Chem Soc, 1994, 116: 10141–10151
Diehl F, Meyer H, Schweig A, Hess B A, Fabian J. 1,2-Dithiete is more stable than 1,2-dithioglyoxal as evidenced by a combined experimental and theoretical IR spectroscopic approach. J Am Chem Soc, 1989, 111: 7651–7653
Schulz R, Schweig A, Hartke K, Koester J. Theory and application of photoelectron spectroscopy. 100. Variable-temperature photoelectron spectral study of 1,3-dithiol-2-one and 4,5-disubstituted 1,3-dithiol-2-ones. Thermal generation of 1,2-dithiete, 3,4-disubstituted 1,2-dithietes, and dialkyl tetrathiooxalates. J Am Chem Soc, 1983, 105: 4519–4528
Vijay D, Sastry G N. Peculiar basis set dependence of the energetics of C2S2H2 isomers. In search of adequate and affordable basis set for routine calculations. J Mol Struct (Theochem), 2005, 732: 71–78
Mucha M, Pagacz M, Mielke Z. Infrared detection of dithioglyoxal from photolysis of 1,3-dithiol-2-one in solid argon and nitrogen. Chem Phys Lett, 2008, 458: 39–43
Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Montgomery J A, Vreven T, Kudin K N, Burant J C, Millam J M, Iyengar S S, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson G A, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda, Y, Kitao O, Nakai H, Klene M, Li X, Knox J E, Hratchian H P, Cross J B, Adamo C, Jaramillo J, Gomperts R, Stratmann R E, Yazyev O, Austin A J, Cammi R, Pomelli C, Ochterski J W, Ayala P Y, Morokuma K, Voth G A, Salvador P, Dannenberg J J, Zakrzewski V G, Dapprich S, Daniels A D, Strain M C, Farkas O, Malick D K, Rabuck A D, Raghavachari K, Foresman J B, Ortiz J V, Cui Q, Baboul A G, Clifford S, Cioslowski J, Stefanov B B, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin R L, Fox D J, Keith T, Al-Laham M A, Peng C Y, Nanayakkara A, Challacombe M, Gill P M W, Johnson B, Chen W, Wong M W, Gonzalez C, Pople J A. Gaussian 03, Revision B, 05. Gaussian Inc, Pittsburgh, PA, 2003
Bearpark M J, Mebel M A. A direct method for the location of the lowest energy point on a potential surface crossing. Chem Phys Lett, 1994, 223: 269–274
Ragazos I N, Robb M A, Bernardi M. Optimization and characterization of the lowest energy point on a conical intersection using an MC-SCF Lagrangian. Chem Phys Lett, 1992, 197: 217–223
He H Y, Fang W H A. CASSCF/MR-CI study toward the understanding of wavelength-dependent and geometrically memorized photodissociation of formic acid. J Am Chem Soc, 2003, 125: 16139–16147
Danovich D, Marian C M, Neuheuser T, Peyerimhoff S D, Shaik S. Spin-orbit coupling patterns induced by twist and pyramidalization modes in C2H4: A quantitative study and a qualitative analysis. J Phys Chem A, 1998, 102: 5923–5936
Isobe H, Yamanaka S, Kuramitsu S, Yamaguchi K. Regulation mechanism of spin-orbit coupling in charge-transfer-induced luminescence of imidazopyrazinone derivatives. J Am Chem Soc, 2008, 130: 132–149
Conti I, Marchioni F, Credi A, Orlandi G, Rosini G, Garavelli M. Cyclohexenylphenyldiazene: A simple surrogate of the azobenzene photochromic unit. J Am Chem Soc, 2007, 129: 3198–3210
Chen H, Li S H. Theoretical study on the photolysis mechanism of 2,3-diazabicyclo[2.2.2]oct-2-ene. J Am Chem Soc, 2005, 127: 13190–13199
Shaik S. Triplet [2+2] cycloadditions. Spin-inversion control of stereoselectivity. J Am Chem Soc, 1979, 101: 3184–3196
Danovich D, Shaik S. Spin-orbit coupling in the oxidative activation of H—H by FeO+. Selection rules and reactivity effects. J Am Chem Soc, 1997, 119: 1773–1786
Su M D. The role of spin-orbit coupling and symmetry in photochemical rearrangements of unsaturated cyclic ketones. Chem Phys, 1996, 205: 277–308
Turro N J. Modern Molecular Photochemistry. Sausalito: University Science Books, 1991
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the “QingLan” Talent Engineering Funds by Tianshui Normal University
Rights and permissions
About this article
Cite this article
Lv, L., Yang, S., Wang, X. et al. Theoretical study of deactivation and isomerization pathways of 1,2-dithiete in excited electronic states. Sci. China Ser. B-Chem. 52, 1176–1185 (2009). https://doi.org/10.1007/s11426-009-0138-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-009-0138-4