Abstract
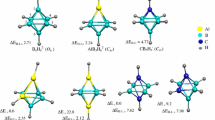
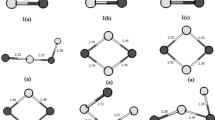
Clusters Al2P2 n− (n = 1–4) were theoretically investigated using density functional theory (DFT) methods at the B3LYP/6-311+G* and B3PW91/6-311+G* levels of theory. The calculated results showed that the planar structure (D 2h symmetry) of Al2P2 n− (n = 1–4) species was the global minimum. And the negative nucleus-independent chemical shift (NICS) value of Al2P2 n− (n = 1–4) species indicated the existence of a ring current in the planar structure (D 2h symmetry). A detailed molecular orbital (MO) analysis revealed that the planar structures (D 2h symmetry) had π aromaticity, which further exhibited the strongly aromatic character for Al2P2 n− (n = 1–4) species.
Similar content being viewed by others
References
Cyranski M K, Krygowski T M, Katritzky A R, Schleyer P v R. To what extent can aromaticity be defined uniquely? J Org Chem, 2002, 67(4): 1333–1338
Nyulászi L. Aromaticity of phosphorus compounds. Chem Rev, 2001, 101: 1229–1246
Kuznetsov A E, Boldyrev A I, Li X, Wang L S. Aromatic mercury clusters in ancient amalgams. J Am Chem Soc, 2001, 123: 8825–8831
Kuznetsov A E, Wang L S, Corbett J D, Boldyrev A I. Aromatic mercury clusters in ancient amalgams. Angew Chem Int Ed, 2001, 40: 3369–3372
Alexandrova A N, Boldyrev A I. Investigations of water monomers in supersaturated NaClO4, LiClO4 and Mg(ClO4)2 droplets using raman spectroscopy. J Phys Chem A, 2003, 107: 554–560
Shetty S, Kanhere D G, Pal S. Antiaromatic Al4Na4 and Al4Na −3 compounds: A theoretical investigation. J Phys Chem A, 2004, 108: 628–631
Kuznetsov A E, Boldyrev A I. A single pi-bond captures 3, 4 and 5 atoms. Chem Phys Lett, 2004, 388(4–6): 452–456
Bldyrev A I, Wang L S. All-metal aromaticity and antiaromaticity. Chem Rev, 2005, 10: 3716–3757
Boldyrev A I, Kuznetsov A E. On the resonance energy in new all-metal aromatic molecules. Inorg Chem, 2002, 4: 532–537
Kuznetsov A E, Boldyrev A I, Zhai H J, Li X, Wang L S. Al 2−6 —Fusion of two aromatic Al −3 units. A combined photoelectron spectroscopy and ab initio study of M+[Al 2−6 ](M = Li, Na, k, Cu, and Au). J Am Chem Soc, 2002, 124: 11791–11801
Mecero J M, Ugalde J M. Sandwich-like complexes based on “all-metal” (Al 2−4 ) aromatic compounds. J Am Chem Soc, 2004, 126: 3380–3381
Chen Z, Corminboeuf C, Heine T, Bohmann J, Schleyer P v R. Do all-metal antiaromatic clusters exist? J Am Chem Soc, 2003, 125: 13930–13931
Li Z W, Zhao C Y, Chen L P. Sandwich complexes of the P 2−4 aromatic ring with the first row transition metal. J Mol Struct (THEOCHEM), 2007, 810: 1–6
Guo L, Wu H S, Jia W H, Jin Z H. Investigation of structures and stabilities of AlmPn and AlmP −n (m + n = 2–6) cluters by DFT. Chin J Chem Phys, 2005, 18: 24–32
Wu H S, Jin Z H, Guo L. First principles study of the structure, electronic state and stability of AlnP −m anions. J Mol Struct (THEOCHEM), 2004, 683: 43–50
Edet F A, Roberto M G, Alexander S A. Structures and electron detachment energies of AlP −2 and Al2P −2 . Chem Phys Lett, 2000, 321: 253–261
Zhao J J, Wang L, Jia J M, Chen X S. Lowest-energy structures of AlnPn (n = 1–9) clusters from density functional theory. Chem Phys Lett, 2007, 443: 29–333
Guo L, Wu H S, Jin Z H. The aluminum phosphides AlmPn (m + n = 2–5) and their anions: Structures, electron affinities and vibrational frequencies. Int J Mass Spectrom, 2005, 240: 149–159
Harry G, Travis R T, Daniel M N. Anion photoelectron spectroscopy of aluminum phosphide clusters. J Phys Chem A, 2001, 105: 6886–6893
Panaghiotis K, Jerzy L. Correlations between bonding, size, and second hyperpolarizability (r) of small semiconductor clusters: Ab initio study on AlnPn clusters with n = 2, 3, 4, 6, and 9. J Chem Phys, 2008, 128: 154323–154333
Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Montgomery J A Jr, Vreven T, Kudin K N, Burant J C, Millam J M, Iyengar S S, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson G A, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox J E, Hratchian H P, Cross J B, Adamo C, Jaramillo J, Gomperts R, Stratmann R E, Yazyev O, Austin A J, Cammi R, Pomelli C, Ochterski J W, Ayala P Y, Morokuma K, Voth G A, Salvador P, Dannenberg J J, Zakrzewski V G, Dapprich S, Daniels A D, Strain M C, Farkas O, Malick D K, Rabuck A D, Raghavachari K, Foresman J B, Ortiz J V, Cui Q, Baboul A G, Clifford S, Cioslowski J, Stefanov B B, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin R L, Fox D J, Keith T, Al-Laham M A, Peng C Y, Nanayakkara A, Challacombe M, Gill P M W, Johnson B, Chen W, Wong M W, Gonzalez C, Pople J A. Gaussian03. Pittsburgh: Gaussian, Inc, 2003
Becke A D. Density-functional thermochemistry III. The role of exact exchange. J Chem Phys, 1993, 98(7): 5648–5652
Lee C, Yang W, Parr R G. Development of the colle-salvetti correlation-energy formula into a functional of the electron density. Phys Rev B, 1988, 37(2): 785–789
Perdew J P, Chevary J A, Vosko S H, Jackson K A, Pederson M R, Singgh D J, Fiolhais C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys Rev B, 1992, 46: 6671–6687
Krishnan R, Binkley J S, Seeger R, Pople J A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J Chem Phys, 1980, 72(1): 650–654
Carpenter J E, Weinhold F. Analysis of the geometry of the hydroxymethyl radical by the “different hybrids for different spins” natural bond orbital procedure. J Mol Struct(THEOCHEM), 1988, 169: 41–62
Wolinski K, Hilton J F, Pulay P. Efficient implementation of the gauge-independent atomic orbital method for NMR chemical shift calculations. J Am Chem Soc, 1990, 112(23): 8251–8260
Ditchfield R. Self-consistent perturbation theory of diamagnetism. I. A gauge-invariant LCAO (linear combination of atomic orbitals) method for NMR chemical shifts. Mol Phys, 1974, 27: 789–807
Sonya B, Grein F. Structure and bonding of III/V compounds X2Y2, with X = B, Al, Ga and Y = N, P, As. J Mol Struct (THEOCHEM), 2005, 757: 137–142
Schleyer P v R, Maerker C, Dransfeld A, Jiao H J, Hommes N J R v E. Nucleus-independent chemical shifts: A simple and efficient aromaticity probe. J Am Chem Soc, 1996, 118(26): 6317–6318
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the 111 Project of China (Grant No. B07012) and the National Natural Science Foundation of China (Grant No. 20773014)
Rights and permissions
About this article
Cite this article
Xu, W., Zhang, Y. & Zhai, L. Structures and aromaticity of the planar Al2P2 n− (n=1–4) clusters. Sci. China Ser. B-Chem. 52, 2237–2242 (2009). https://doi.org/10.1007/s11426-009-0117-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-009-0117-9