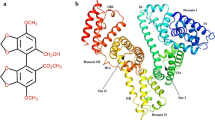
Abstract
The binding of caffeine to human serum albumin (HSA) under physiological conditions has been studied by the methods of fluorescence, UV-vis absorbance and circular dichroism (CD) spectroscopy. The mechanism of quenching of HSA fluorescence by caffeine was shown to involve a dynamic quenching procedure. The number of binding sites n and apparent binding constant K b were measured by the fluorescence quenching method and the thermodynamic parameters ΔH, ΔG, ΔS were calculated. The results indicate that the binding is mainly enthalpy-driven, with van der Waals interactions and hydrogen bonding playing major roles in the reaction. The distance r between donor (HSA) and acceptor (caffeine) was obtained according to the Förster theory of non-radiative energy transfer. Synchronous fluorescence, CD and three-dimensional fluorescence spectroscopy showed that the microenvironment and conformation of HSA were altered during the reaction.
Similar content being viewed by others
References
Barone J J, Roberts H R. Caffeine consumption. Food Chem Toxicol, 1996, 34: 119–129
Lieberman H R, Wurtman R J, Garfield G S, Roberts C, Coviella I L G. The effects of low doses of caffeine on human performance and mood. Psychopharmacology, 1987, 92: 308–312
Jarvis M J. Does caffeine intake enhance absolute levels of cognitive performance? Psychopharmacology, 1993, 110: 45–52
Fitzgerald G K, Axe M J, Snyder-Mackler L. The Efficacy of perturbation training in nonoperative anterior cruciate ligament rehabilitation programs for physically active individuals. Phys Ther, 2000, 80: 128–140
La Vecchia C, Ferraroni M, Negri E, D’Avanzo, B. Decarli A, Levi F, Franceschi S. Coffee consumption and digestive tract cancers. Cancer Res, 1989, 49: 1049–1051
Joesoef M R, Beral V, Rolfs R T, Aral S O, Cramer D W. Are caffeinated beverages risk factors for delayed conception? Lancet, 1990, 335: 136–137
Pedersen A O, Mensberg K L, Kragh-Hansen U. Effects of ionic strength and pH on the binding of medium-chain fatty acids to human serum albumin. Eur J Biochem, 1995, 233: 395–405
Curry S, Mandelkow H, Brick P, Franks N. Crystal structure of human serum albumin complexed with fatty acid reveals an asymmetric distribution of binding sites. Nat Struct Biol, 1998, 5: 827–835
Bhattacharya A A, Grüne T, Curry S. Crystallographic analysis reveals common modes of binding of medium and long-chain fatty acids to human serum albumin. J Mol Biol, 2000, 303: 721–732
He X M, Carter D C. Atomic structure and chemistry of human serum albumin. Nature, 1992, 358: 209–215
Carter D C, Ho J X. Structure of serum albumin. Adv Protein Chem, 1994, 45: 153–203
Carter D C, Chang B, Ho J X, Keeling K, Krishnasami Z. Preliminary crystallographic studies of four crystal forms of serum albumin. Eur J Biochem, 1994, 226: 1049–1052
Perry J L, Il’ichev Y V, Kempf V R, McClendon J, Park G, Manderville R A, Ruker F, Dockal M, Simon J D. Binding of ochratoxin A derivatives to human serum albumin. J Phys Chem B, 2003, 107: 6644–6647
Bian Q Q, Liu J Q, Tian J N, Hu Z D. Binding of genistein to human serum albumin demonstrated using tryptophan fluorescence quenching. Internat J Biol Macromol, 2004, 34: 27–279
Olson R E, Christ D D. Plasma protein binding of drugs. Ann Rep Med Chem, 1996, 31: 327–336
Flarakos J, Morand K L, Vouros P. High-throughput solution-based medicinal library screening against human serum albumin. Anal Chem, 2005, 77: 1345–1353
Lakowicz J R. Principles of Fluorescence Spectroscopy. 2nd ed. New York: Plenum Press, 1999. 237–265
Lakowicz J R, Weber G. Quenching of fluorescence by oxygen. A probe for structural fluctuations in macromolecules. Biochemistry, 1973, 12: 4161–4170
Hu Y J, Liu Y, Hou A X, Zhao R M, Qu X S, Qu S S. Studies on the interaction between rare-earth salts of heteropoly EuHSiMo10-W2O40·25H2O and bovine serum albumin (in Chinese). Acta Chimica Sinica, 2004, 62: 1519–1523
Hu Y J, Liu Y, Wang J B, Xiao X H, Qu S S. Study of the interaction between monoammonium glycyrrhizinate and bovine serum albumin. J Pharm Biomed Anal, 2004, 36: 915–919
Ross P D, Subramanian S. Thermodynamics of protein association reaction: forces contribution to stability. Biochemistry, 1981, 20: 3096–3102
Forster T. Intermolecular energy migration and fluorescence. Ann Phys, 1948, 2: 55–75
Sklar L A, Hudson B S, Simoni R D. Conjugated polyene fatty acids as fluorescent probes: Synthetic phospholipid membrane studies. Biochemistry, 1977, 16: 819–828
Long C, King E J, Sperry W M. Biochemists’ Handbook. London: E & F N Spon Ltd, 1961. 84
Weiss S. Fluorescence spectroscopy of single biomolecules. Science, 1999, 283: 1676–1683
Hu Y J, Liu Y, Sun T Q, Bai A M, Lu J Q, Pi Z B. Binding of anti-inflammatory drug cromolyn sodium to bovine serum albumin. Int J Biol Macromol, 2006, 39: 280–285
Miller J N. Recent advances in molecular luminescence analysis. Proc Anal Div Chem Soc, 1979, 16: 203–208
Klajnert B, Bryszewska M. Fluorescence studies on PAMAM dendrimers interactions with bovine serum albumin. Bioelectrochemistry, 2002, 55: 33–35
Kamat B P, Seetharamappa J. In vitro study on the interaction of mechanism of tricyclic compounds with bovine serum albumin. J Pharm Biomed Anal, 2004, 35: 655–664
Cui F L, Fan J, Li J P, Hu Z D. Interactions between 1-benzoyl-4-p-chlorophenyl thiosemicarbazide and serum albumin: Investigation by fluorescence spectroscopy. Bioorg Med Chem, 2004, 12: 151–157.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, Q., Li, C., Hu, Y. et al. Study of caffeine binding to human serum albumin using optical spectroscopic methods. Sci. China Ser. B-Chem. 52, 2205–2212 (2009). https://doi.org/10.1007/s11426-009-0114-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-009-0114-z