Abstract
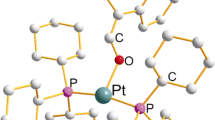
The anion coordination complex, [Cl⊂Pt(bpt)4]Cl (bpt=N,N′-bis(3-pyridylmethyl)-2-thiourea), was synthesized and studied by X-ray crystal structure analysis, NMR and FAB mass spectra. In the solid state, the Pt(bpt)4 anion receptor adopts a cone conformation to bind the chloride anion through hydrogen bonds and electrostatic interaction in which the four branches of the thiourea ligands bind the chloride anion to form N-H⋯Cl− hydrogen bonds (3.49–3.81 Å). The entraped chloride anion is situated above the Pt(II) center at 3.52 Å. Further second-sphere coordination assemby from the Pt(bpt)4 core with 8 zinc(II) tetraphenylporphyrins (ZnPr) is discussed.
Similar content being viewed by others
References
Lehn J M. Supramolecular Chemistry: Concepts and Perspectives. Weinheim: VCH, 1995
Bianchi A. Bowman J K, García-España E (eds). Supramolecular Chemistry of Anions. New York: Wiley-VCH, 1997
Gale P A. Anion receptor chemistry: Highlights from 1999. Coord Chem Rev, 2001, 213: 79–128
Gale P A. Anion coordination and anion-directed assembly: Highlights from 1997 and 1998. Coord Chem Rev, 2000, 199: 181–233
Bowman-James K. Alfred Werner revisited: The coordination chemistry of anions. Acc Chem Res, 2005, 38: 671–678
Beer P D. Gale P A. Anion recognition and sensing: The state of the art and future perspectives. Angew Chem Int Ed, 2001, 40: 487–516
Vilar R. Anion-templated synthesis. Angew Chem Int Ed, 2003, 42: 1460–1477
Snowden T S, Anslyn E V. Anion recognition: Synthetic receptors for anions and their application in sensors. Curr Opin Chem Bio, 1999, 3: 740–746
Martnez-Mez R, Sancenn F. Fluorogenic and chromogenic chemosensors and reagents for anions. Chem Rev, 2003, 103: 4419–4476
Bondy C R, Gale P A, Loeb S J. Metal-organic anion receptors: Arranging urea hydrogen-bond donors to encapsulate sulfate ions. J Am Chem Soc, 2004, 126(16): 5030–5031
Custelcean R, Remy P, Bonnesen P V, Jiang D, Moyer B A. Sulfate recognition by persistent crystalline capsules with rigidified hydrogen-bonding cavities. Angew Chem Int Ed, 2008, 47: 1866–1870
Wu B, Liang J, Yang J, Jia C, Yang X J, Zhang H, Tang N, Janiak C. Sulfate ion encapsulation in caged supramolecular structures assembled by second-sphere coordination. Chem Commun, 2008, 48: 1762–1764
Yu S Y, Huang H., Liu H B. Modular cavity-tunable self-assembly of molecular bows and crowns as structural analogues of calix[3]arenes. Angew Chem Int Ed, 2003, 42: 686–690
Li S H, Huang H P, Yu S Y. Self-assembly of coordination molecular baskets as inorganic analogues of cyclotriveratrylenes (CTV). Dalton Trans, 2005, 14: 2346–2349
Liu L X, Huang H P, Li X, Sun Q F, Sun C R, Li Y Z, Yu S Y. Coordination molecular hats binding acetonitrile via C-H⋯π interactions. Dalton Trans, 2008, 12: 1544–1546
Battaglia L P, Bonamartini C A, Vidoni T M E. Tetrakis (N,N′-diethylthiourea) platinum(II) chloride monohydrate, C20H50Cl21N8OPtS4. Cryst Struct Commun, 1975, 4: 361
Sanders J K M. Comprehensive Supramolecular Chemistry (Chair ed Lehn J M). Oxford: Pergamon, 1996, Vol 9. 132–162
Xu H, Ng D P. Construction of Subphthalocyanine-Porphyrin and Subphthalocyanine-Phthalocyanine Heterodyads through Axial Coordination. Inorg Chem, 2008, 47: 7921–7927
Shmilovits M, Vinodu M, Goldberg I. Coordination polymers of tetra(4-carboxyphenyl)porphyrins sustained by tetrahedral zinc ion linkers. Crys Grow Des, 2004, 3: 633–638
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China (Grant Nos. 50673098 & 20772152), Renmin University of China, State Key Laboratory of Structure Chemistry (Grant No. 20080052)
Rights and permissions
About this article
Cite this article
Li, X., Li, H., Yu, S. et al. Anion coordination complex [Cl⊂Pt(bpt)4]Cl (bpt=N,N′-bis(3-pyridylmethyl)-2-thiourea). Sci. China Ser. B-Chem. 52, 471–474 (2009). https://doi.org/10.1007/s11426-009-0099-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-009-0099-7