Abstract
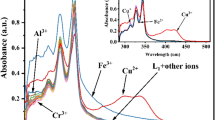
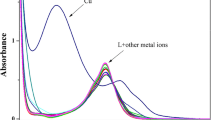
A new perylene diimide (PDI) ligand (1) functionalized with a dipicolylethylenediamine (DPEN) moiety was synthesized and first used as a colorimetric and fluorometric dual-channel sensor to specifically detect the presence of Cu2+ over a wide range of other cations. The solution of 1 (10 μmol/L) upon addition of Cu2+ displayed distinguishing pink color compared with other cations including K+, Ni2+, Ca2+, Mn2+, Na+, Sr2+, Zn2+, Co2+, Cd2+, Mg2+, Cr3+, Ag+, and Ba2+, indicating the sensitivity and selectivity of 1 to Cu2+. Thus, the advantage of this assay is that naked-eye detection of Cu2+ becomes possible. Moreover, among these metal ions investigated, only Cu2+ quenched more than half fluorescent intensity of 1. The ESI-TOF spectrum of a mixture of 1 and CuCl2 in combination of the fluorescence titration spectra of 1 (10 μmol/L) upon addition of various amounts of Cu2+ revealed the formation of a 2:1 metal-ligand complex through the metal coordination interaction.
Similar content being viewed by others
References
Prodi L, Bolletta F, Montalti M, Zaccheroni N. Luminescent chemosensors for transition metal ions. Coord Chem Rev, 2000, 205(1): 59–83
De Silva A P, Fox D B, Huxley A J M, Moody T S. Combining luminescence, coordination and electron transfer for signalling purposes. Coord Chem Rev, 2000, 205(1): 41–57
Millhauser G L. Copper binding in the prion protein. Acc Chem Res, 2004, 37(2): 79–85
Gaggelli E, Kozlowski H, Valensin D, Valensin G. Copper homeostasis and neurodegenerative disorders (alzheimer’s, prion, and parkinson’s diseases and amyotrophic lateral sclerosis). Chem Rev, 2006, 106(5): 1995–2044
Kim H J, Hong J, Hong A, Ham S, Lee J H, Kim J S. Cu2+-induced intermolecular static excimer formation of pyrenealkylamine. Org Lett, 2008, 10(10): 1963–1966
Yu M M, Li Z X, Wei L H, Wei D H, Tang M S. A 1,8- naphthyridine-based fluorescent chemodosimeter for the rapid detection of Zn2+ and Cu2+. Org Lett, 2008, 10(10): 5115–5118
Xu Z, Xiao Y, Qian X, Cui J, Cui D. Ratiometric and selective fluorescent sensor for CuII based on internal charge transfer (ICT). Org Lett, 2005, 7(5): 889–892
Lee S K, Zu Y, Herrmann A Y, Geerts Müllen K, Bard A J. Electrochemistry, spectroscopy and electrogenerated chemiluminescence of perylene, terrylene, and quaterrylene diimides in aprotic solution. J Am Chem Soc, 1999, 121(14): 3513–3520
Abe T, Nagai K, Kabutomori S, Kaneko M, Tajiri A, Norimatsu T. An organic photoelectrode working in the water phase: Visible-light- induced dioxygen evolution by a perylene derivative/cobalt phthalocyanine bilayer. Angew Chem Int Ed, 2006, 45(17): 2778–2781
Sugiyasu K, Fujita N, Shinkai S. Visible-light-harvesting organogel composed of cholesterol-based perylene derivatives. Angew Chem Int Ed, 2004, 43(10): 1229–1233
Lee H N, Xu Z, Kim S K, Swamy K M K, Kim Y, Kim S J, Yoon J. Pyrophosphate-selective fluorescent chemosensor at physiological pH: Formation of a unique excimer upon addition of pyrophosphate. J Am Chem Soc, 2007, 129(13): 3828–3829
Ling K Q, Sayre L M. A dopaquinone model that mimics the water addition step of cofactor biogenesis in copper amine oxidases. J Am Chem Soc, 2005, 127(13): 4777–4784
Goldsmith C R, Lippard S J. 6-Methylpyridyl for pyridyl substitution tunes the properties of fluorescent zinc sensors of the zinpyr family. Inorg Chem, 2006, 45(2): 555–561
Kiyose K, Kojima H, Urano Y, Nagano T. Development of a ratiometric fluorescent zinc ion probe in near-infrared region, based on tricarbocyanine chromophore. J Am Chem Soc, 2006, 128(20): 6548–6549
Goldsmith C R, Lippard S J. Analogues of zinpyr-1 provide insight into the mechanism of zinc sensing. Inorg Chem, 2006, 45(13): 6474–6478
Kawabata E, Kikuchi K, Urano Y, Kojima H, Odani A, Nagano T. Design and synthesis of zinc—Selective chelators for extracellular applications. J Am Chem Soc, 2005, 127(3), 818–819
de Silva A P, Gunaratne H QN, Lynch P L M. Luminescence and charge transfer. Part 4. ‘On-off’ fluorescent PET (photoinduced electron transfer) sensors with pyridine receptors: 1,3-diaryl-5-pyridyl-4,5-dihydropyrazoles. J Chem Soc, Perkin Trans 2, 1995, 2(4): 685–690
de Silva A P, Gunaratne H QN, McCoy C P. Direct visual indication of pH windows: ‘off-on-off’ fluorescent PET (photoinduced electron transfer) sensors/switches. Chem Commun, 1996, (21): 2399–2400
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China (Grant Nos. 20872101 & 20772086)
Rights and permissions
About this article
Cite this article
Yan, L., Yang, L., Lan, J. et al. A new perylene diimide-based colorimetric and fluorescent sensor for selective detection of Cu2+ cation. Sci. China Ser. B-Chem. 52, 518–522 (2009). https://doi.org/10.1007/s11426-009-0041-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-009-0041-z