Abstract
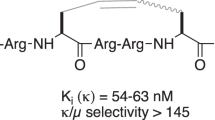
An unnatural amino acid, β-[6′-(N, N-dimethyl)amino-2′-naphthoyl]alanine (Ald) showing polarity-sensitive fluorescence characteristics, was synthesized. A thorough Ald-scan of dynorphin A (Dyn A), the putative endogenous ligand for. opioid receptors, was then performed. Replacement of the amino acid residues in positions 5, 8, 10, 12 or 14 of Dyn A(1–13)-NH2 with Ald resulted in compounds that had almost equal κ binding affinity compared with that of the parent compound; on the other hand, substitution of residues in position 1 or 4 with Ald decreased κ-receptor binding affinity. These results indicate that Tyr and Phe in Dyn A are very important for maintaining its κ-opioid activity. Evidence from receptor binding assay clearly displays that [Ald5]Dyn A(1–13)-NH2 is a highly selective κ-opioid receptor agonist. An evaluation of the interaction of Ald-containing Dyn A(1–13)-NH2 analogues with SDS and DPC micelles was also performed. Interestingly, [Ald1]Dyn A(1–13)-NH2 and [Ald4]Dyn A(1–13)-NH2 showed quite different fluorescence emission maxima in SDS and DPC micelles. This indicates that both peptides are sensitive to electronic properties of the polar surface of the micelles.
Similar content being viewed by others
References
Chavkin C, James I F, Goldstein A. Dynophin is a specific endogenous ligand of the Kappa opioid receptor. Science, 1982, 215(4531): 413–415
Long J B, Rigmonti D D, Costa B, Rice K C, Martinez-Arizala A. Dynorphin A-induced rat hindlimb paralysis and spinal cord injury are not altered by the kappa opioid antagonist nor-binaltorphimine. Brain Res, 1989, 497(1): 155–162
Hruby V J, Agnes R S. Conformation-activity relationships of opioid peptides with selective activities at opioid receptors. Biopolymers, 1999, 51(6): 391–410
Chavkin C, James I F, Goldstein A. Specific receptor for the opioid peptide dynorphin: Structure-activity relationships. Proc Nat Acad, Sci USA, 1981, 78(10): 6541–6547
Cohen B E, McAnaney T B, Park E S, Jan Y N, Boxer S G, Jan L Y. Probing protein electrostatics with a synthetic fluorescent amino acid. Science, 2002, 296, 1700–1703
Vazquez M E, Nitz M, Stehn J, Yaffe M B, Imperiali B. Fluorescent caged phosphoserine peptides as probes to investigate phosphorylation-dependent protein associations. J Am Chem Soc, 2003, 125 (34): 10150–10151
Paton W D M. The action of morphine and related substances on contraction and on acetylcholine output of coaxially stimulated guinea-pig ileum. Br J Pharmacol Chemother, 1957, 12(1): 119–127
Henderson G, Hughes J, Kosterlitz H W. A new example of a morphine-sensitive neuro-effector junction: Adrenergic transmission in the mouse vas deferens. Br J Pharmacol, 1972, 46(4): 764–766
Schiller P W, Lipton A, Horrobin D F, Bondanzsky M. Unsulfated C-terminal 7-peptide of cholecystokinin: A new ligand of the opiate receptor. Biochem Biophys Res Commun, 1978, 85(4): 1332–1338
DiMaio J, Nguyen T M D, Lemieux C, Schiller P W. Synthesis and biological evaluation of a metazocine-containing enkephalinamide. Evidence for nonidentical roles of the tyramine moiety in opiates and opioid peptides. J Med Chem, 1982, 25(12): 1432–1438
Kosterlitz H W, Watt A J. Kinetic parameters of narcotic agonists and antagonists, with particular reference to N-allylnoroxymorphone (naloxone). Br J Pharmacol Chemother, 1968, 33(2): 266–276
Cheng Y C, Prusoff W H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 percent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol, 1973, 22(23): 3099–3102
Chen H R, Guo X K, Zhong X B. Syntheses of aladan and [Ald6] loloatin C and study on their fluorescent properties. Chin J Chem, 2006, 24(10): 1411–1417
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China (Grant No. 30672560) and the Ascended Project for Natural Scientific Research of Universities in Guangdong Province (Grant No. 05Z012).
Rights and permissions
About this article
Cite this article
Chen, H., Yang, Y. & Weng, J. Aladan scanning: The structure-activity relationship of dynorphin A. Sci. China Ser. B-Chem. 52, 338–343 (2009). https://doi.org/10.1007/s11426-008-0150-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-008-0150-0