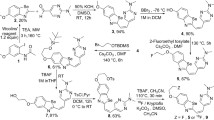
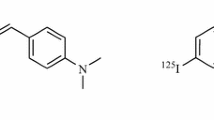
Abstract
As amyloid β (Aβ) is at the centre of pathogenesis of Alzheimer’s disease (AD), Aβ aggregate-specific probes for in vivo studies of Aβ are potentially important for the early diagnosis and the assessment of new treatment strategies in the AD brain by noninvasive imaging. Several series of compounds derived from Congo red (CR) and Thioflavin T (ThT) have been evaluated as potential probes for the Aβ imaging. They include a diversity of core structures contributing to their affinities to Aβ. Small-molecule inhibitors were known to inhibit the formation of Aβ oligomers and fibrils. This inhibition has to be performed in such a way that these inhibitors bind to Aβ in the binding channel where Aβ-binding probes should sit. Therefore, several of them were used as novel core structures to develop Aβ probes, with their derivatives exhibiting good Aβ affinities. This approach will facilitate the design of a variety of candidates for Aβ probe molecules and anti-aggregation-therapeutic drugs. Moreover, the finding of Aβ probes with diverse core structures recognized by binding sites on Aβs will likely provide a promising perspective for the design of 99mTc-labeled probe-derived molecules.
Similar content being viewed by others
References
Selkoe D J. The origins of Alzheimer disease: A is for amyloid. JAMA, 2000, 283: 1615–1617
Kang J, Lemaire H G, Unterbeck A, Salbaum J M, Masters C L, Grzeschik K H, Multhaup G, Beyreuthe K, Muller-Hill B. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature, 1987, 325: 733–736
Harper J D, Lansbury P T Jr. Models of amyloid seeding in Alzheimer’s disease and scrapie: Mechanistic truths and physiological consequences of the time-dependent solubility of amyloid proteins. Annu Rev Biochem, 1997, 66: 385–407
Yankner B A, Duffy L K, Kirschner D A. Neurotrophic and neurotoxic effects of amyloid beta protein: Reversal by tachykinin neuropeptides. Science, 1990, 250: 279–282
Harkany T, Abraham I, Konya C, Nyakas C, Zarandi M, Penke B, Luiten P G. Mechanisms of beta-amyloid neurotoxicity: Perspectives of pharmacotherapy. Rev Neurosci, 2000, 11: 329–382
Walsh D M, Klyubin I, Fadeeva J V, Cullen W K, Anwyl R, Wolfe M S, Rowan M J, Selkoe D J. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature, 2002, 416: 535–539
Gong Y S, Chang L, Viola K L, Lacor P N, Lambert M P, Finch C E, Krafft G A, Klein W L. Alzheimer’s disease-affected brain: Presence of oligomeric Aβ ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proc Natl Acad Sci USA, 2003, 100:10417–10422
Tabner B J, Turnbull S, El Agnaf O M, Allsop D. Formation of hydrogen peroxide and hydroxyl radicals from Aβ and R-synuclein as a possible mechanism of cell death in Alzheimer’s disease and Parkinson’s disease. Free Radical Biol Med, 2002, 32: 1076–1083
Barnham K J, Masters C L, Bush A I. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov, 2004, 3: 205–214
Kawahara M, Kuroda J. Molecular mechanism of neurodegeneration induced by Alzheimer’s beta-amyloid protein: Channel formation and disruption of calcium homeostasis. Brain Res Bull, 2000, 53: 389–397
McGeer E G, McGeer P L. Brain inflammation in Alzheimer disease and the therapeutic implications. Curr Pharm Design, 1999, 5: 821–836
Moore A H, O’Banion M K. Neuroinflammation and anti-inflammatory therapy for Alzheimer’s disease. Adv Drug Delivery Rev, 2002, 54: 1627–1656
Barrow C J. Advances in the development of Abeta-related therapeutic strategies for Alzheimer’s disease. Drug News Perspect, 2002, 15:102–109
Masters C L, Cappai R, Barnham K J, Villemagne V L. Molecular mechanisms for Alzheimer’s disease: Implications for neuroimaging and therapeutics. J Neurochem, 2006, 97: 1700–1725
Nordberg A. PET imaging of amyloid in Alzheimer’s disease. Lancet Neurol, 2004, 3: 519–527
Norfray J F, Provenzale J M. Alzheimer’s disease: Neuropathologic findings and recent advances in imaging. Am J Roentgenol, 2004, 182:3–13
Wang Y, Mathis C A, Huang G F, Holt D P, Debnath M L, Klunk W E. Synthesis and 11C-labelling of (E,E)-1-(30,40-dihydroxystyryl)-4-(30-methoxy-40-hydroxystyryl) benzene for PET imaging of amyloid depositsy. J Label Compd Radiopharm, 2002, 45: 647–664
Klunk W E, Wang Y M, Huang G F, Debnath M L, Holt D P, Mathis C A. Uncharged thioflavin-T derivatives bind to amyloid-beta protein with high affinity and readily enter the brain. Life Sci, 2001, 69:1471–1484
Mathis C A, Wang Y, Klunk W E. Imaging β-amyloid plaques and neurofibrillary tangles in the aging human brain. Curr Pharm Design, 2004, 10: 1469–1492
Styren S D, Hamilton R L, Styren G C, Klunk W E. X-34, a fluorescent derivative of congo red. A novel histochemical stain for Alzheimer’s disease pathology. J Histochem Cytochem, 2000, 48: 1223–1232
Kung H F, Lee C W, Zhuang Z P, Kung M P, Hou C, Plössl K. Novel stilbenes as probes for amyloid plaques. J Am Chem Soc, 2001, 123: 12740–12741
Lee C W, Kung M P, Hou C, Kung H F. Dimethylamino-fluorenes: Ligands for detecting β-amyloid plaques in the brain. Nucl Med Biol, 2003, 30: 573–580
Zhuang Z P, Kung M P, Hou C, Plössl K, Kung H F. Biphenyls labeled with technetium 99m for imaging β-amyloid plaques in the brain. Nucl Med Biol, 2005, 32: 171–184
Zhuang Z P, Kung M P, Hou C, Skovronsky D M, Gur T L, Plössl K, Trojanowski J Q, Lee V M Y, Kung H F. Radioiodinated styrylbenzenes and thioflavins as probes for amyloid aggregates. J. Med Chem, 2001, 44: 1905–1914
Zhuang Z P, Kung M P, Hou C, Plössl K, Skovronsky D, Gur T L, Trojanowski J Q, Lee V M Y, Kung H F. IBOX (2-(4′-dimethylaminophenyl)-6-iodobenzoxazole): A ligand for imaging amyloid plaques in the brain. Nucl Med Biol, 2001, 28: 887–894
Kung M P, Hou C, Zhuang Z P, Zhang B, Skovronsky D, Trojanowski J Q, Lee V M Y, Kung H F. IMPY: An improved thioflavin-T derivative for in vivo labeling of β-amyloid plaques. Brain Res, 2002, 956:202–210
Ono M, Kung M P, Hou C, Kung H F. Benzofuran derivatives as Aβ-aggregate-specific imaging agents for Alzheimer’s disease. Nucl Med Biol, 2002, 29: 633–642
Agdeppa E D, Kepe V, Liu J, Flores-Torres S, Satyamurthy N, Petric A, Cole G M, Small G W, Huang S C, Barrio J R. Binding characteristics of radiofluorinated 6-dialkylamino-2-naphthylethylidene derivatives as positron emission tomography imaging probes for beta-amyloid plaques in Alzheimer’s disease. J Neurosci, 2001, 21:RC189 1–5
Chandra R, Kung M P, Kung H F. Design, synthesis, and structure-activity relationship of novel thiophene derivatives for β-amyloid plaque imaging. Bioorg Med Chem Lett, 2006, 16: 1350–1352
Chandra R, Oya S, Kung M P, Hou C, Jin L W, Kung H F. New diphenylacetylenes as probes for positron emission tomographic imaging of amyloid plaques. J Med Chem, 2007, 50: 2415–2423
Qu W, Kung M P, Hou C, Oya S, Kung H F. Quick assembly of 1,4-diphenyltriazoles as probes targeting β-amyloid aggregates in alzheimer’s disease. J Med Chem, 2007, 50: 3380–3387
Lockhart A, Ye L, Judd D B, Merritt A T, Lowe P N, Morgenstern J L, Hong G, Gee A D, Brown J. Evidence for the presence of three distinct binding sites for the Thioflavin T class of Alzheimer’s disease PET imaging agents on β-amyloid peptide fibrils. J Biol Chem, 2005, 280: 7677–7684
LeVine H. Multiple ligand binding sites on Aβ(1–40) fibrils. Amyloid, 2005, 12: 5–14
Agdeppa E D, Kepe V, Liu J, Small G W, Huang S C, Petric A, Satyamurthy N, Barrio J R. 2-dialkylamino-6-acylmalononitrile substituted naphthalenes (DDNP analogs): Novel diagnostic and therapeutic tools in Alzheimer’s disease. Mol Imaging Biol, 2003, 5: 404–417
Krebs M R H, Bromley E H C, Donald A M. The binding of thioflavin-T to amyloid fibrils: Localization and implications. J Struct Biol, 2005, 149: 30–37
Chen X J. QSAR and primary docking studies of trans-stilbene (TSB) series of imaging agents for β-amyloid plaques. J Mol Struc-Theochem, 2006, 763: 83–89
Ono K, Yoshiike Y, Takashima A, Hasegawa K, Naiki H, Yamada M. Potent anti-amyloidogenic and fibril-destabilizing effects of polyphenols in vitro: Implications for the prevention and therapeutics of Alzheimer’s disease. J Neurochem, 2003, 87: 172–181
Ono K, Hasegawa K, Naiki H, Yamada M. Curcumin has potent anti-amyloidogenic effects for Alzheimer’s β-amyloid fibrils in vitro. J Neurosci Res, 2004, 75: 742–750
Yang F S, Lim G P, Begum A N. Curcumin inhibits formation of amyloid β oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem, 2005, 280: 5892–5901
Ono M, Yoshida N, Ishibashi K, Haratake M, Arano Y, Mori H, Nakayama M. Radioiodinated flavones for in vivo imaging of β-amyloid plaques in the brain. J Med Chem, 2005, 48: 7253–7260
Ono M, Maya Y, Haratake M, Ito K, Mori H, Nakayama M. Aurones serve as probes of β-amyloid plaques in Alzheimer’s disease. Biochem Bioph Res Co, 2007, 361: 116–121
Ono M, Maya Y, Haratake M, Nakayama M. Synthesis and characterization of styrylchromone derivatives as β-amyloid imaging agents. Bioorg Med Chem, 2007, 15: 444–450
Ono M, Hori M, Haratake M, Tomiyama T, Mori H, Nakayama M. Structure-activity relationship of chalcones and related derivatives as ligands for detecting of β-amyloid plaques in the brain. Bioorg Med Chem, 2007, 15: 6388–6396
Ryu E K, Choe Y S, Lee K H, Choi Y, Kim B T. Curcumin and dehydrozingerone derivatives: synthesis, radiolabeling, and evaluation for β-Amyloid plaque imaging. J Med Chem, 2006, 49: 6111–6119
Lee I, Choe YS, Kim D H, Lee K H, Choi Y, Choi JY, Kim B T. Synthesis and evaluation of RS-0406 derivatives for beta-amyloid plaque and neurofibrillary tangle imaging. J Label Compd Radio Pharm, 2007, 50: S409
Gazit E. A possible role for π-stacking in the selfassembly of amyloid fibrils. FASEB J, 2002, 16: 77–83
Makin O S, Atkins E, Sikorski P, Johansson J, Serpell L C. Molecular basis for amyloid fibril formation and stability. Proc Natl Acad Sci USA, 2005, 102: 315–320
Tjernberg L, Callaway D, Tjernberg A, Hahne S, Lilliehook C, Terenius L, Thyberg J, Norstedt C. A molecular model of Alzheimer amyloid β-peptide fibril formation. J Biol Chem, 1999, 274: 12619–12625
Mage P P. Molecular simulation of the primary and secondary structures of the Aβ(1–42) peptide of Alzheimer’s disease. Med Res Rev, 1998, 18: 403–430
Thomas T, Nadackal G T, Thomas K. Aspirin and nonsteroidal anti-inflammatory drugs inhibit amyloid-β aggregation. Neuroreport, 2001, 12: 3263–3267
Agdeppa E D, Kepe V, Petric A, Satyamurthy N, Liu J, Huang S C, Small G W, Cole G M, Barrio J R. In vitro detection of (S)-naproxen and ibuprofen binding to plaques in the Alzheimer’s brain using the positron emission tomography molecular imaging probe 2-(1-{6-[(2-[18F] fluoroethyl) (methyl) amino]-2napthyl}ethylidene) malononitrile. Neurosci, 2003, 117: 723–730
Hirohata M, Ono K, Naiki H, Yamada M. Non-steroidal anti-inflammatory drugs have anti-amyloidogenic effects for Alzheimer’s β-amyloid fibrils in vitro. Neuropharmacology, 2005, 49: 1088–1099
Lee V M Y. Amyloid binding ligands as Alzheimer’s disease therapies. Neurobiol Aging, 2002, 23: 1039–1042
Stains C I, Mondal K, Ghosh I. Molecules that target beta-amyloid. ChemMedChem, 2007, 2: 1674–1692
Collier T L, Waterhouse R N, Kassiou M. Imaging sigma receptors:application in drug development. Curr Pharm Design, 2007, 13:51–72
Johannsen B, Pietzsch H J. Development of technetium-99m-based CNS receptor ligands: have there been any advances? Eur J Nucl Med, 2002, 29: 263–275
Zhuang Z P, Kung M P, Hou C, Ploessl K, Kung H F. Biphenyls labeled with technetium 99m for imaging β-amyloid plaques in the brain. Nucl Med Biol, 2005, 32: 171–184
Serdons K, Verduyckt T, Cleynhens J, Terwinghe C, Mortelmans L, Bormans G, Verbruggen A. Synthesis and evaluation of a 99mTc-BAT-phenylbenzothiazole conjugate as a potential in vivo tracer for visualization of amyloid β. Bioorg Med Chem Lett, 2007, 17: 6086–6090
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China (Grant No. 20471011)
Rights and permissions
About this article
Cite this article
Duan, X., Liu, B. Aβ-binding molecules: Possible application as imaging probes and as anti-aggregation agents. Sci. China Ser. B-Chem. 51, 801–807 (2008). https://doi.org/10.1007/s11426-008-0075-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-008-0075-7