Abstract
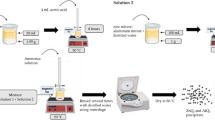
A novel strong-fluorescent hydrotalcite-like compound (Al-HTLc) was synthesized by coprecipitation. In the sample, the content of aluminum(III) in the layers was decreased to a proper value. The Al3+ ions coordinated with 8-hydroxyquinolines (8-HQ) which were dispersed into the anions in the interlayer region. The sample was characterized by XRD, XPS, FT-IR, TG-DSC, UV-Vis, and fluorescence spectroscopy; its composition and structure were also determined. The results indicate that the sample can emit fluorescence (487 nm) with a strong fluorescence intensity (4.9×105 (a.u.)). The fluorescent lifetime and fluorescence quantum yield of Al-HTLc were measured to be 21.24 ns and 67%, respectively, higher than those of pure 8-hydroxyquinoline aluminum (Alq3). The result of TG-DSC measurement clearly shows the enhanced thermal stability of Al-HTLc compared with that of MgAl-LDH and pure Alq3. Al-HTLc may be used as a novel luminescent functional material.
Similar content being viewed by others
References
Canavi F, Trifiro F, Vaccari A. Hydrotalcite-type anionic clays: Preparation, properties and applications. J Catal Today, 1991, 11: 173–301
Xing Y, Li D Q, Ren L L, Evans D G, Duan X. Assembly and structural characteristics of supramolecular salicylate-pillared hydrotalcites. Acta Chim Sin (in Chinese), 2003, 61(2): 267–272
Gou G J, Ma P H, Chu M X. Dynamics of intercalation of B4O5(OH)2− 4 anion into layered double hydroxides intercalated by Cl− anion. Chem J Chin Univ (in Chinese), 2005, 26: 497–502
Latterini L, Nocchetti M, Aloisi G G, Costantino U, Elisei F. Organized chromophores in layered inorganic matrices. Inor Chim Acta, 2006,7: 48–61
Bhaumik A, Samanta S, Mal N K. Efficient removal of arsenic from polluted ground water by using a layered double hydroxide exchanger. Indian J Chem Section α-Inorg Bio-lnorg Phys Theor & Anal Chem, 2005, 44(7): 1406–1409
Yang P P, Wu T H. The structure, synthesize and catalysis application of hydrotalcite. Petrochem Tech & Appl (in Chinese), 2005, 23(1): 61–66
Prescott H A, Li Z J, Kemnitz E. Application of calcined Mg-AI hydrotalcites for Michael additions: An investigation of catalytic activity-and acid-base properties. J Catalysis, 2005, 234(1): 119–130
Wei M, Xu X Y, Wang X R, Li F, Zhang H, Lu Y L, Pu M, David G. Evans, Duan X. Study on the photochromism of Ni-Al layered double hydroxides containing nitrate anions. Eur J Inorg Chem, 2006, 14: 2831–2838
You Y W, Zhao H T, Vance G F. Hybrid organic-inorganic derivatives of layered double hydroxides and dodecylbenzenesulfonate: Preparation and adsorption characteristics. J Mater Chem, 2002, 12: 907–912
Tang C W, Vanslyke S A. Organic electroluminescenct diodes. Appl Phys Lett, 1987, 51(12): 913–915
Mohd Zobir bin Hussein, Tan K H. Synthesis and properties of layered organic-inorganic hybrid material: Zn-Al layered double hydroxide-dioctyl sulfosuccinate nanocomposite. J Nanopart Rese, 2000, 2: 293–298
Velu S, Ramkumar V, Narayanan A. Effect of interlayer anions on the physicochemical properties of zinc-aluminum hydrotalcite-like compounds. J Mater Sci, 1997, 32: 957–964
Wang C X, Yu Z Q, Li C, Wang B X, Huang X H. Synthesis and fluorescence properties of bis (2-phenyl-8-hydroxyquinolato) zinc. Chin J Lumin (in Chinese), 2004, 25(4): 414–418
Hao Y Y, Hao H T, Wang H, Zhou H F, Liu X G, Xu B S. Preparation and characterization of 2(8-hydroxyquinoline)-2(phenol) zirconium thin film. Spectro and Spect Anal (in Chinese), 2004, 24(12): 1524–1527
Ma L D. Altitude Structure Analysis (in Chinese). Shanghai: Fudan Press, 2002. 157–165
Ouyang X H, Zeng H P, Xie Y. Synthesis and photoluminescence properties of 8-hydroxyquinoline derivatives and their metal ion complexes. Chin J Organ Chem (in Chinese), 2007, 27(3): 402–408
Zhu L J, Guo C C. An improved method for preparation of high-purity tris-(8-hydroxyquinoline) aluminum. Huaxue Shiji (in Chinese), 2004, 26(6): 369–370
Chen G Z, Huang X Zh, Zheng Zh Zh. Fluorescence Analytical method (in Chinese). 2nd ed. Beijing: Science Press, 1990. 15–17
Qiu L Z, Qu B J. Preparation and characterization of surfactant-free polystyrene/layered double hydroxide exfoliated nanocomposite via soap-free emulsion polymerization. J Coll Interf Sci, 2006, 301: 347–351
Zou Y D, Huang X B, Lin W Z. The photoluminescence characteristics study on Alq3 in polymer films. Acta Phys-Chim Sin (in Chinese), 1999, 15(4): 375–380
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China (Grant No. 50272014), the Key Item of Natural Scientific Foundation of Fujian Province (Grant No. 2001F005) and the Key Nano Special Item of Fujian Province (Grants No. 2005HZ01-5)
Rights and permissions
About this article
Cite this article
Zhang, W., Chen, H. Preparation and characterization of a novel strong-fluorescent hydrotalcite-like compound (Al-HTLc). Sci. China Ser. B-Chem. 51, 834–841 (2008). https://doi.org/10.1007/s11426-008-0038-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-008-0038-z