Abstract
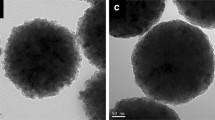
The interaction between stabilizers and nanoparticles is one of the important factors to prepare stable magnetic fluids. The magnetic nano-size Fe3O4 core with single domain and the average grain size around 8–12 nm were prepared by chemical precipitation method. The O/Fe molar ratio of the particle surface was measured by X-ray photoelectron spectroscopy (XPS). The heat effects of stabilizers adsorption on nanoparticles were measured by solution calorimetry. The excess amount of oxygen was possibly the result of the hydroxygen formed on the surface of the nanoparticles. The heat effects showed that compounds containing carboxyl groups can be adsorbed chemically on magnetite by forming chemical bonds. The other stabilizers involving NH-groups, such as polyethylene-imine, can be adsorbed physically. The exothermic value is about half of the former case.
Similar content being viewed by others
References
Yoza B, Arakaki A, Matsunaga T. DNA extraction using bacterial magnetic particles modified with hyperbranched polyamidoamine dendrimer. J Biotechnol, 2003, 101: 219–228
Xu X Q, Shen H, Xu J R, Xu J, Li X J, Xiong X M. Core-shell structure and magnetic properties of magnetite magnetic fluids stabilized with dextran. Appl Surf Sci, 2005, 252: 494–500
Astalan A P, Ahrentorp F, Johansson C, Larsson K, Krozer A. Biomolecular reactions studied using changes in Brownian rotation dynamics of magnetic particles. Biosens Bioelectron, 2004, 19: 945–951
Arol A I, Aydogan A. Recovery enhancement of magnetite fines in magnetic separation. Colloid Surface A: Physicochem Eng Aspects, 2004, 232: 151–154
Ma Z Y, Guan Y P, Liu H Zh. Superparamagnetic silica nanoparticles with immobilized metal affinity ligands for protein adsorption. J Magn Magn Mater, 2006, 301: 469–477
Ma Z Y, Liu X Q, Guan Y P, Liu H Zh. Synthesis of magnetic silica nanospheres with metal ligands and application in affinity separation of proteins. Colloid Surface A, 2006, 275: 87–91
Morel J P, Marmier N, Hurel C, Morel-Desrosiers N. Effect of temperature on the acid-base properties of the alumina surface: Microcalorimetry and acid-base titration experiments. J Colloid Interf Sci, 2006, 298(2): 773–779
Yu H G, Liu Y, Tan Zh Ch, Dong J X, Zou T J, Huang X M, Qu S Sh. A solution-reaction isoperibol calorimeter and standard molar enthalpies of formation of Ln(hq)2Ac (Ln = La, Pr). Thermochim Acta, 2003, 401(2): 217–224
Blanco-MantecÓn M, O’Grady K. Interaction and size effects in magnetic nanoparticles. J Magn Magn Mater, 2006, 296: 124–133
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China (Grant No. 50476039), and Guangdong Provincial Department of Science and Technology (Grant No. 2004A10-703001)
Rights and permissions
About this article
Cite this article
Xia, J., Shen, H., Zhang, W. et al. Surface chemistry of nanoscale Fe3O4 dispersed in magnetic fluids. Sci. China Ser. B-Chem. 50, 754–758 (2007). https://doi.org/10.1007/s11426-007-0136-3
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11426-007-0136-3