Abstract
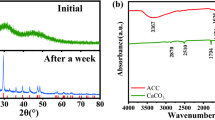
To probe the scale inhibition mechanisms, calcium carbonate scale occurring before and after the addition of scale inhibitors was collected. The results from scale SEM confirm that, without scale inhibitor, calcium carbonate scale shows rhombohedron and hexagon, which are the characteristic feathers of calcite. After addition of inhibitors, morphology of scale is changed, and the more efficient the scale inhibitor is, the more greatly the morphology is modified. To elucidate the scale constitute, they were further analyzed by FT-IR, XRD. Besides calcite, vaterite and aragonite occur in calcium carbonate scale after addition of inhibitors, and the higher scale inhibition efficiency is, the more vaterite presents in scale. It can be concluded that the alteration of morphology is ascribed to the change of crystal form. There are three stages in the crystallizing process including occurrence and disappearing of unstable phase, occurrence and disappearing of metastable phase, development of stable phase. Without scale inhibitors, metastable phases usually transform into stable phase, thus the main constitute of formed scale is calcite. When scale inhibitors are added, both formation and transformation of metastable phases are inhibited, which results in the occurrence of aragonite and vaterite. From the fact that more vaterite presents in scale with a more efficient scale inhibitor added, we can see that the function of scale inhibitor is realized mainly by controlling the crystallizing process at the second stage.
Similar content being viewed by others
References
Takeshi O, Toshio S, Kiyoshi S. The formation and transformation mechanism of calcium carbonate in water. Geochim Cosmochim Acta, 1987, 151(4): 2757–2767
Plummer L N, Busenberg E. The solubilities of calcite, aragonite and vaterite in CO2-H2O solutions between 0 and 90°C, and an evaluation of the aqueous model for the system CaCO3-CO2-H2O. Geochim Cosmochim Acta, 1982, 46(3): 1011–1040
Brecevic L. Solubility of amorphous calcium carbonate. J Cryst Growth, 1989, 98(2): 504–510
Kralj D, Brecevic L, Kontrec J. Vaterite growth and dissolution in aqueous solution (III): Kinetics of transformation. J Cryst Growth, 1997, 177(4): 248–257
Sawada K. The mechanism of crystallization and transformation of calcium carbonates. Pure Appl Chem, 1997, 69(5): 921–928
Nikos S, Petros G K. The transformation of vaterite to calcite: Effect of the conditions of the solutions in contact with the mineral phase. J Cryst Growth, 1998, 191(4): 783–790
Yang Q F, Liu Y Q, Gu A Z. Investigation of calcium carbonate scaling inhibition and scale morphology by AFM. J Colloid Interf Sci, 2001, 240(2): 608–621
Nancollas G H, Sawada K. Formation of scale of calcium carbonate polymorphs: The influence of magnesium ion and inhibitors. J Petrol Technol, 1982, 34(2): 645–652
Xyla A G, Mikroyannidis J, Koutsoukos P G. The inhibition of calcium carbonate precipitation in aqueous media by organophosphorus compound. J Colloid Interf Sci, 1992, 153(2): 537–551
Burns K, Wu Y-T, Grant C S. Mechanisms of calcite dissolution using environmentally benign polyaspartic acid: A rotating disk study. Langmuir, 2003, 19(14): 5669–5679
Dimirtra G K, Petros G K. The calcitic marble/water interface: Kinetics of dissolution and inhibition with potential implications in stone conservation. Langmuir, 2003, 19(14): 5691–5699
Gal J Y, Bollinger J C, Tolosa H. Calcium carbonate solubility: A reappraisal of scale formation and inhibition. Talanta, 1996, 43(9): 1497–1509
Apostolis K, Nikos S. Effect of inorganic phosphate ions on the spontaneous precipitation of vaterite and on the transformation of vaterite to calcite. J Cryst Growth, 1999, 204(2): 83–90
Abdel A N, Sawda K. Inhibition of adhesion and precipitation of CaCO3 by aminopolyphosphonate. J Cryst Growth, 2003, 256(2): 188–200
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, G., Ge, J., Sun, M. et al. Investigation of scale inhibition mechanisms based on the effect of scale inhibitor on calcium carbonate crystal forms. SCI CHINA SER B 50, 114–120 (2007). https://doi.org/10.1007/s11426-007-0010-3
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11426-007-0010-3