Abstract
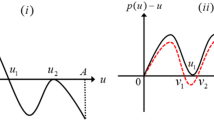
How to prevent and control the outbreak of mosquito-borne diseases, such as malaria, dengue fever and Zika, is an urgent worldwide public health problem. The most conventional method for the control of these diseases is to directly kill mosquitoes by spraying insecticides or removing their breeding sites. However, the traditional method is not effective enough to keep the mosquito density below the epidemic risk threshold. With promising results international, the World Mosquito Program’s Wolbachia method is helping to reduce the occurrence of diseases transmitted by mosquitoes. In this paper, we introduce a generalized discrete model to study the dynamics of the Wolbachia infection frequency in mosquito populations where infected mosquitoes are impulsively released. This generalized model covers all the relevant existing models since 1959 as some special cases. After summarizing known results of discrete models deduced from the generalized one, we put forward some interesting open questions to be further investigated for the periodic impulsive releases.
Similar content being viewed by others
References
Bian G, Joshi D, Dong Y, et al. Wolbachia invades Anopheles stephensi populations and induces refractoriness to Plasmodium infection. Science, 2013, 340: 748–751
Caspari E, Watson G S. On the evolutionary importance of cytoplasmic sterility in mosquitoes. Evolution, 1959, 13: 568–570
Cohen J. Dengue may bring out the worst in Zika. Science, 2017, 355: 1362
Fine P E M. Vectors and vertical transmission: An epidemiologic perspective. Ann N Y Acad Sci, 1975, 266: 173–194
Fine P E M. On the dynamics of symbiote-dependent cytoplasmic incompatibility in culicine mosquitoes. J Invertebr Pathol, 1978, 31: 10–18
Hedges L M, Brownlie J C, O’Neill S L, et al. Wolbachia and virus protection in insects. Science, 2008, 322: 702
Hoffmann A A, Montgomery B L, Popovici J, et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature, 2011, 476: 454–457
Hoffmann A A, Turelli M, Harshman L G. Factors affecting the distribution of cytoplasmic incompatibility in Drosophila simulans. Genetics, 1990, 126: 933–948
Hu L, Tang M, Wu Z, et al. The threshold infection level for Wolbachia invasion in random environments. J Differential Equations, 2019, 266: 4377–4393
Huang M, Yu J, Hu L, et al. Qualitative analysis for a Wolbachia infection model with diffusion. Sci China Math, 2016, 59: 1249–1266
Laven H. Eradication of Culex pipiens fatigans through cytoplasmic incompatibility. Nature, 1967, 216: 383–384
Mcmeniman C J, Lane R V, Cass B N, et al. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science, 2009, 323: 141–144
Rasgon J L, Styer L M, Scott T W. Wolbachia-induced mortality as a mechanism to modulate pathogen transmission by vector arthropods. J Med Entomol, 2003, 40: 125–132
Schwartz L M, Halloran M E, Durbin A P, et al. The dengue vaccine pipeline: Implications for the future of dengue control. Vaccine, 2015, 33: 3293–3298
Shi Y, Yu J. Wolbachia infection enhancing and decaying domains in mosquito population based on discrete models. J Biol Dyn, 2020, 14: 679–695
Stanaway J D, Shepard D S, Undurraga E A, et al. The global burden of dengue: An analysis from the Global Burden of Disease Study 2013. Lancet Infect Dis, 2016, 16: 712–723
Turelli M, Hoffmann A A. Rapid spread of an inherited incompatibility factor in California Drosophila. Nature, 1991, 353: 440–442
Turelli M, Hoffmann A A. Cytoplasmic incompatibility in Drosophila simulans: Dynamics and parameter estimates from natural populations. Genetics, 1995, 140: 1319–1338
Turelli M, Hoffmann A A. Microbe-induced cytoplasmic incompatibility as a mechanism for introducing transgenes into arthropod populations. Insect Mol Biol, 1999, 8: 243–255
Walker T, Johnson P H, Moreira L A, et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature, 2011, 476: 450–453
Waltz E. US reviews plan to infect mosquitoes with bacteria to stop disease. Nature, 2016, 533: 450–451
Wang Y, Liu X, Li C, et al. A survey of insecticide resistance in Aedes albopictus (Diptera: Culicidae) during a 2014 dengue fever outbreak in Guangzhou, China. J Econ Entomol, 2017, 110: 239–244
Xi Z, Khoo C C, Dobson S L. Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science, 2005, 310: 326–328
Yu J. Modelling mosquito population suppression based on delay differential equations. SIAM J Appl Math, 2018, 78: 3168–3187
Yu J, Li J. Global asymptotic stability in an interactive wild and sterile mosquito model. J Differential Equations, 2020, 269: 6193–6215
Yu J, Zheng B. Modeling Wolbachia infection in mosquito population via discrete dynamical models. J Difference Equ Appl, 2019, 25: 1549–1567
Zhang X, Liu Q, Zhu H. Modeling and dynamics of Wolbachia-infected male releases and mating competition on mosquito control. J Math Biol, 2020, 81: 243–276
Zheng B, Yu J, Li J. Modeling and analysis of the implementation of the Wolbachia incompatible and sterile insect technique for mosquito population suppression. SIAM J Appl Math, 2021, 81: 718–740
Zheng B, Yu J, Xi Z, et al. The annual abundance of dengue and Zika vector Aedes albopictus and its stubbornness to suppression. Ecol Modell, 2018, 387: 38–48
Zheng X, Zhang D, Li Y, et al. Incompatible and sterile insect techniques combined eliminate mosquitoes. Nature, 2019, 572: 56–61
Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant Nos. 11971127, 12071095 and 11631005) and the Changjiang Scholars Program and Program for Innovative Research Team in University (Grant No. IRT_16R16).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zheng, B., Li, J. & Yu, J. One discrete dynamical model on the Wolbachia infection frequency in mosquito populations. Sci. China Math. 65, 1749–1764 (2022). https://doi.org/10.1007/s11425-021-1891-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11425-021-1891-7
Keywords
- Wolbachia infection frequency
- cytoplasmic incompatibility
- mosquito population
- release strategy
- periodic impulsive release