Abstract
Purpose
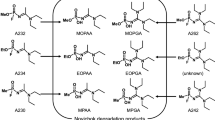
The detection of hydrolysis products of nerve agents (alkyl methylphosphonic acids; RMPAs) in biological samples from victims is important to confirm exposure to nerve agents. However, analysis of RMPAs is difficult due to their high hydrophilicity. The aim of this study was to develop ion chromatography–tandem mass spectrometry (IC–MS/MS) methods using commercially available equipment and columns to analyze RMPAs in human urine and serum with high sensitivity and without using complicate techniques.
Methods
A Dionex IonPac AS11-HC anion-exchange column was used to analyze six RMPAs (MPA, EMPA, IMPA, iBuMPA, CHMPA, and PMPA). For pretreatments of biological fluids, we developed two pretreatment methods (Method 1: dilution and ultrafiltration; Method 2: removal of chloride ions with Ag cartridges).
Results
Six RMPAs including highly hydrophilic methylphosphonic acid and ethyl methylphosphonic acid could be analyzed with sufficient retention times and peak shape. The detection limits of RMPAs were improved using Dionex OnGuard II Ba/Ag/H cartridges and MetaSEP IC–Ag cartridges (urine: 0.5–5 ng/mL; serum: 1–5 ng/mL). These methods were also applied to the test samples for the Organisation for the Prohibition of Chemical Weapons Biomedical Proficiency Tests.
Conclusions
RMPAs could be sufficiently analyzed by IC–MS/MS. In addition, the limits of detection were superior to those obtained in our previous study involving LC–MS/MS or derivatization–LC–MS/MS method. For analysis of biological samples, an appropriate pretreatment method can be chosen according to the amount of sample available for analysis and expected RMPA concentrations.





Similar content being viewed by others
References
Kassa J (2002) Review of oximes in the antidotal treatment of poisoning by organophosphorus nerve agents. J Toxicol Cli Toxicol 40:803–816
Organization for the prohibition of chemical weapons, chemical weapon convention, http://www.opcw.org. Accessed 15 Nov 2021.
Seto Y, Tsunoda N, Kataoka M, Tsuge K, Nagano T (1999). In: Tu AA, Gaffield A (eds) Natural and selected synthetic toxins: biological implications. American Chemical Society, Washington, DC, pp 318–332
Noort D, Hulst AG, Platenburg DHJM, Polhuijs M, Benschop HP (1998) Quantitative analysis of O-isopropyl methylphosphonic acid in serum samples of Japanese citizens allegedly exposed to sarin: estimation of internal dosage. Arch Toxicol 72:671–675
Tsuchihashi H, Katagi M, Nishikawa M, Tatsuno M (1998) Identification of metabolites of nerve agent VX in serum collected from a victim. J Anal Toxicol 22:383–388
Yang YC, Baker JA, Ward JR (1992) Decontamination of chemical warfare agents. Chem Rev 92:1729–1743
Seto Y (2011) Research and development of on-site decontamination system for biological and chemical warfare agents. J Health Sci 57:311–333
Seto Y (2015). In: Gupta RC (ed) Handbook of the toxicology of chemical warfare agents, 2nd edn. Elsevier, Amsterdam, pp 897–914
Black RM, Read RW (2013) Biological markers of exposure to organophosphorus nerve agents. Arch Toxicol 87:421–437
Munro NB, Talmage SS, Griffin GD, Waters LC, Watson AP, King JF, Hauschild V (1999) The sources, fate, and toxicity of chemical warfare agent degradation products. Environ Health Perspect 107:933–974
Black RM, Read RW, Muir B (2003) Derivatisation reactions in the chromatographic analysis of chemical warfare agents and their degradation products. J Chromatogr A 1000:253–281
Jang YJ, Kim K, Tsay OG, Atwood DA, Churchill DG (2015) Destruction and detection of chemical warfare agents. Chem Rev 115:PR1–PR76
Black RM, Read RW (1997) Application of liquid chromatography-atmospheric pressure chemical ionisation mass spectrometry, and tandem mass spectrometry, to the analysis and identification of degradation products of chemical warfare agents. J Chromatogr A 759:79–92
Hamelin EI, Schulze ND, Shaner RL, Coleman RM, Lawrence RJ, Crow BS, Jakubowski EM, Johnson RC (2014) Quantitation of five organophosphorus nerve agent metabolites in serum using hydrophilic interaction liquid chromatography and tandem mass spectrometry. Anal Bioanal Chem 406:5195–5202
Røen BT, Sellevåg SR, Dybendal KE, Lundanes E (2014) Trace determination of primary nerve agent degradation products in aqueous soil extracts by on-line solid phase extraction-liquid chromatography-mass spectrometry using ZrO2 for enrichment. J Chromatogr A 1329:90–97
Lee JY, Lee YH (2014) Rapid screening and determination of nerve agent metabolites in human urine by LC–MS/MS. J Anal Chem 69:909–916
Otsuka M, Tsuge K, Miyaguchi H, Uchiyama M (2018) Analysis of degradation products of nerve agents via post-pentafluorobenzylation liquid chromatography–tandem mass spectrometry. J Chromatogr A 1577:31–37
Røen BT, Sellevag SR, Lundanes E (2014) Quantification of nerve agent biomarkers in human serum and urine. Anal Chem 86:11833–11840
Mawhinney DB, Hamelin EH, Fraser R, Silva SS, Pavlopoulos AJ, Kobelski RJ (2007) The determination of organophosphonate nerve agent metabolites in human urine by hydrophilic interaction liquid chromatography tandem mass spectrometry. J Chromatogr B 852:235–243
Kingery AF, Allen HE (1994) Ion chromatographic separation of closely related nerve agent degradation products using an organic modifier to provide selectivity. Anal Chem 66:155–159
Piao H, Marx RB, Schneider S, Irvine DA, Staton J (2005) Analysis of VX nerve agent hydrolysis products in wastewater effluents by ion chromatography with amperometric and conductivity detection. J Chromatogr A 1089:65–71
Katagi M, Nishikawa M, Tatsuno M, Tsuchihashi H (1997) Determination of the main hydrolysis products of organophosphorus nerve agents, methylphosphonic acids, in human serum by indirect photometric detection ion chromatography. J Chromatogr B 698:81–88
Tokarz JAIII, Ginter JM, Shefcheck KJ (2017) Ion chromatography (IC). In: Vanninen P (ed) Recommended operating procedures for analysis in the verification of chemical disarmament. University of Helsinki, Finland, pp 405–415
Baygildiev T, Vokuev M, Ogorodnikov R, Braun A, Rybalchenko I, Rodin I (2019) Simultaneous determination of organophosphorus nerve agent markers in urine by IC-MS/MS using anion-exchange solid-phase extraction. J Chromatogr B 1132:121815
Seto Y, Tachikawa M, Kanamori-Kataoka M, Sasamoto K, Ochiai N (2017) Target analysis of tert-butyldimethylsilyl derivatives of nerve agent hydrolysis products by selectable one-dimensional or two-dimensional gas chromatography-mass spectrometry. J Chromatogr A 1501:99–106
Dubey V, Velikeloth S, Sliwakowski M, Mallard G (2009) Official proficiency tests of the organisation for the prohibition of chemical weapons: current status and future directions. Accred Qual Assur 14:431–437
Quality System Document of the OPCW: Work Instruction for the Reporting of the Results of the OPCW Biomedical Proficiency Tests (QDOC_LAB_WI_BioPT04 (Issue 1, Revision 1, dated 28 December 2016))
Method Detection Limit - Frequent Questions, United States Environmental Protection Agency. https://www.epa.gov/cwa-methods/method-detection-limit-frequent-questions. Accessed 17 May 2022
Evaluation of the results of the fifth official opcw biomedical proficiency test. https://www.opcw.org. Accessed 14 December 2021.
Baygildiev TM, Vokuev MF, Braun AV, Yashkir VA, Rybalchenko IV, Rodin IA (2021) Identification of 2-(diethylamino)ethylthiol dipeptide (Cys-Pro) adduct as biomarker of nerve agents VR and CVX in human plasma using liquid chromatography-high-resolution tandem mass spectrometry. Anal Bioanal Chem 413:1905–1916
Nakajima T, Sasaki K, Ozawa H, Sekijima Y, Morita H, Fukushima Y, Yanagisawa N (1998) Urinary metabolites of sarin in a patient of the Matsumoto sarin incident. Arch Toxicol 72:601–603
Carlson M, Thompson RD (2000) Analyte loss due to membrane filter adsorption as determined by high-performance liquid chromatography. J Chromatogr Sci 38:77–83
Funding
This work was supported by Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Early Career Scientists [grant number 20K18990 to M.O.]. We thank ThinkSCIENCE Inc. (http://www.thinkscience.co.jp/) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by M.O. All authors read and approved to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
Collection of the urine samples was performed in line with the principles of the Declarations of Helsinki and approved by the ethics committee of the National Research Institute of Police Science.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Otsuka, M., Miyaguchi, H. Analysis of degradation products of nerve agents in biological fluids by ion chromatography–tandem mass spectrometry. Forensic Toxicol 41, 71–80 (2023). https://doi.org/10.1007/s11419-022-00633-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11419-022-00633-x