Abstract
Purpose
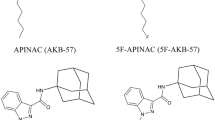
Adamantan-1-yl 1-pentyl-1H-indazole-3-carboxylate (APINAC), a new synthetic cannabinoid, was recently isolated from a dietary supplement and identified in our laboratories. The metabolism and pharmacokinetics of APINAC were studied in vitro and in vivo with a rat model.
Methods
APINAC (2.0 μM) was incubated with rat liver microsomes (RLMs) for up to 90 min to determine its phase I metabolic profile. APINAC was also administered to rats at a dose of 5 mg/kg intravenously (i.v.) or 10 mg/kg per os (p.o.). Blood samples were collected at specific time points, and urine samples were also collected for 1 day following APINAC administration. The analyses were conducted by both high- and low-resolution liquid chromatography–tandem mass spectrometry.
Results
Although APINAC was rapidly metabolized by RLMs (t 1/2, 15.2 ± 0.4 min), in vivo pharmacokinetic experiments in rats revealed moderate to long half-lives [11.3 h (i.v.) and 3.8 h (p.o.)]. A total of 22 APINAC metabolites could be detected in the RLMs and rat urine. APINAC was predominantly metabolized via ester hydrolysis to carboxylic acids M1 (with hydroxylation) and M18 (without hydroxylation), representative markers for APINAC intake. Hydroxylation of APINAC, with or without subsequent oxidation and glucuronidation, was observed in the case of the other metabolites.
Conclusions
The diagnosis of illegal APINAC intake can be realized through the detection of several characteristic APINAC metabolites in human urine and/or APINAC itself in blood.









Similar content being viewed by others
References
Nutt D, King LA, Saulsbury W, Blakemore C (2007) Development of a rational scale to assess the harm of drugs of potential misuse. Lancet 369:1047–1053
van Amsterdam J, Nutt D, Phillips L, van den Brink W (2015) European rating of drug harms. J Psychopharmacol 29:655–660
Pompei P, Di Bonaventura MVM, Cifani C (2016) The “legal highs” of novel drugs of abuse. J Drug Abuse 2:2. doi:10.21767/2471-853X.100025
Babor TF, McRee BG, Kassebaum PA, Grimaldi PL, Ahmed K, Bray J (2007) Screening, brief intervention, and referral to treatment (SBIRT) toward a public health approach to the management of substance abuse. Subst Abuse 28:7–30
Higgins ST, Silverman K, Heil SH (eds) (2008) Contingency management in substance abuse treatment. Guilford Press, New York London
EMCDDA (2016) Synthetic cannabinoids in Europe. http://www.emcdda.europa.eu/topics/pods/synthetic-cannabinoids. Accessed 18 Dec 2016
Hermanns-Clausen M, Kneisel S, Szabo B, Auwärter V (2013) Acute toxicity due to the confirmed consumption of synthetic cannabinoids: clinical and laboratory findings. Addiction 108:534–544
Seely KA, Lapoint J, Moran JH, Fattore L (2012) Spice drugs are more than harmless herbal blends: a review of the pharmacology and toxicology of synthetic cannabinoids. Prog Neuropsychopharmacol Biol Psychiatry 39:234–243
Forrester MB, Kleinschmidt K, Schwarz E, Young A (2012) Synthetic cannabinoid and marijuana exposures reported to poison centers. Hum Exp Toxicol 31:1006–1011
Young AC, Schwarz E, Medina G, Obafemi A, Feng SY, Kane C, Kleinschmidt K (2012) Cardiotoxicity associated with the synthetic cannabinoid, K9, with laboratory confirmation. Am J Emerg Med 30:e1325–e1327
Chimalakonda KC, Seely KA, Bratton SM, Brents LK, Moran CL, Endres GW, James LP, Hollenberg PF, Prather PL, Radominska-Pandya A, Moran JH (2012) Cytochrome P450-mediated oxidative metabolism of abused synthetic cannabinoids found in K2/Spice: identification of novel cannabinoid receptor ligands. Drug Metab Dispos 40:2174–2184
Sobolevsky T, Prasolov I, Rodchenkov G (2012) Detection of urinary metabolites of AM-2201 and UR-144, two novel synthetic cannabinoids. Drug Test Anal 4:745–753
Scheidweiler KB, Jarvis MJ, Huestis MA (2015) Nontargeted SWATH acquisition for identifying 47 synthetic cannabinoid metabolites in human urine by liquid chromatography–high-resolution tandem mass spectrometry. Anal Bioanal Chem 407:883–897
Uchiyama N, Kawamura M, Kikura-Hanajiri R, Goda Y (2012) Identification of two new-type synthetic cannabinoids, N-(1-adamantyl)-1-pentyl-1H-indole-3-carboxamide (APICA) and N-(1-adamantyl)-1-pentyl-1H-indazole-3-carboxamide (APINACA), and detection of five synthetic cannabinoids, AM-1220, AM-2233, AM-1241, CB-13 (CRA-13), and AM-1248, as designer drugs in illegal products. Forensic Toxicol 30:114–125
Drug Enforcement Administration, Department of Justice (2016) Schedules of controlled substances: placement of UR-144, XLR11, and AKB48 into Schedule I. Final rule. Fed Regist 81:29142–29145
Lee JH, Park HN, Leem TS, Jeon JH, Cho S, Lee J, Baek SY (2017) Identification of new synthetic cannabinoid analogue APINAC (adamantan-1-yl 1-pentyl-1H-indazole-3-carboxylate) with other synthetic cannabinoid MDMB (N)-Bz-F in illegal products. Forensic Toxicol 35:45–55
Savchuk S, Appolonova S, Pechnikov A, Rizvanova L, Shestakova K, Tagliaro F (2017) In vivo metabolism of the new synthetic cannabinoid APINAC in rats by GC–MS and LC–QTOF-MS. Forensic Toxicol 35:359–368
Gandhi AS, Zhu M, Pang S, Wohlfarth A, Scheidweiler KB, Liu HF, Huestis MA (2013) First characterization of AKB-48 metabolism, a novel synthetic cannabinoid, using human hepatocytes and high-resolution mass spectrometry. AAPS J 15:1091–1098
Diao X, Carlier J, Zhu M, Pang S, Kronstrand R, Scheidweiler KB, Huestis MA (2017) In vitro and in vivo human metabolism of a new synthetic cannabinoid NM-2201 (CBL-2201). Forensic Toxicol 35:20–32
Wohlfarth A, Gandhi AS, Pang S, Zhu M, Scheidweiler KB, Huestis MA (2014) Metabolism of synthetic cannabinoids PB-22 and its 5-fluoro analog, 5F-PB-22, by human hepatocyte incubation and high-resolution mass spectrometry. Anal Bioanal Chem 406:1763–1780
Wintermeyer A, Möller I, Thevis M, Jübner M, Beike J, Rothschild MA, Bender K (2010) In vitro phase I metabolism of the synthetic cannabimimetic JWH-018. Anal Bioanal Chem 398:2141–2153
Holm NB, Pedersen AJ, Dalsgaard PW, Linnet K (2014) Metabolites of 5F-AKB-48, a synthetic cannabinoid receptor agonist, identified in human urine and liver microsomal preparations using liquid chromatography high-resolution mass spectrometry. Drug Test Anal 7:199–206
Vikingsson S, Josefsson M, Green H (2015) Identification of AKB-48 and 5F-AKB-48 metabolites in authentic human urine samples using human liver microsomes and time of flight mass spectrometry. J Anal Toxicol 39:426–435
Castaneto MS, Scheidweiler KB, Gandhi A, Wohlfarth A, Klette KL, Martin TM, Huestis MA (2014) Quantitative urine confirmatory testing for synthetic cannabinoids in randomly collected urine specimens. Drug Test Anal 7:483–493
Hutter M, Broecker S, Kneisel S, Auwärter V (2012) Identification of the major urinary metabolites in man of seven synthetic cannabinoids of the aminoalkylindole type present as adulterants in ‘herbal mixtures’ using LC–MS/MS techniques. J Mass Spectrom 47:54–65
Acknowledgements
This research was supported by a research grant (15182MFDS523) from the Ministry of Food and Drug Safety (MFDS) in Korea and by research grants from Kangwon National University. We thank the Central Laboratory of Kangwon National University for providing technical assistance with the spectroscopic experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not describe any studies that include human participants performed by any of the authors. All experimental protocols involving the animals in this study were approved by the Institutional Animal Care and Use Committee of Kangwon National University (permit number: KW-161219-2).
Rights and permissions
About this article
Cite this article
Hwang, J., Hwang, J., Ganganna, B. et al. Metabolic and pharmacokinetic characterization of a new synthetic cannabinoid APINAC in rats. Forensic Toxicol 36, 88–101 (2018). https://doi.org/10.1007/s11419-017-0387-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11419-017-0387-4