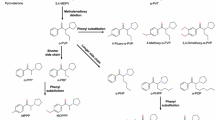
Abstract
The aim of this review is to present the chemical aspects, pharmacology, acute toxicities, and metabolisms of α-pyrrolidinophenone derivatives, a new group of synthetic cathinones. Compared to other synthetic cathinones, α-pyrrolidinophenone derivatives have high lipophilicity due to the pyrrolidine ring substitution at the nitrogen atom, resulting in higher blood–brain barrier permeability. To date, some acute intoxication and fatal cases involving α-pyrrolidinophenone derivatives have been reported, and the symptoms induced by their high dosages are due to central nervous system and cardiovascular toxicities. Based on the previous metabolism studies, reduction of the β-ketone moiety to the corresponding alcohol metabolites and oxidation to the 2′′-oxo metabolites are the main metabolic pathways observed among α-pyrrolidinophenone derivatives. In addition to such pathways, specific metabolic pathways like hydroxylation followed by oxidation of the 4′-methyl group, O-demethylation of the 4′-methoxyl group, and demethylenation followed by O-methylation of the 3′,4′-methylenedioxy group can be observed for the corresponding ring-substituted compounds.



Similar content being viewed by others
Abbreviations
- bk-MBDB:
-
2-Methylamino-1-(3,4-methylenedioxyphenyl)butan-1-one
- bk-MDAs:
-
3′,4′-Methylenedioxy-β-ketoamphetamines
- bk-MDEA:
-
2-Ethylamino-1-(3,4-methylenedioxyphenyl)propan-1-one
- Cathinone:
-
S-(−)-2-Amino-1-phenylpropan-1-one
- MDAs:
-
3′,4′-Methylenedioxyamphetamines
- MDPBP:
-
3′,4′-Methylenedioxy-α-pyrrolidinobutiophenone
- MDPHP:
-
3′,4′-Methylenedioxy-α-pyrrolidinohexiophenone
- MDPPP:
-
3′,4′-Methylenedioxy-α-pyrrolidinopropiophenone
- MDPV (MDPVP):
-
3′,4′-Methylenedioxy-α-pyrrolidinovalerophenone
- Mephedrone:
-
4′-Methylmethcathinone
- Methylone:
-
3′,4′-Methylenedioxymethcathinone
- MOPBP:
-
4′-Methoxy-α-pyrrolidinobutiophenone
- MOPHP:
-
4′-Methoxy-α-pyrrolidinohexiophenone
- MOPPP:
-
4′-Methoxy-α-pyrrolidinopropiophenone
- MOPVP:
-
4′-Methoxy-α-pyrrolidinovalerophenone
- MPBP:
-
4′-Methyl-α-pyrrolidinobutiophenone
- MPHP:
-
4′-Methyl-α-pyrrolidinohexiophenone
- MPPP:
-
4′-Methyl-α-pyrrolidinopropiophenone
- Naphyrone (O-2482):
-
Naphthylpyrovalerone
- PBP:
-
α-Pyrrolidinobutiophenone
- PHP:
-
α-Pyrrolidinohexiophenone
- PMA:
-
p-Methoxyamphetamine
- PMEA:
-
p-Methoxyethylamphetamine
- PMMA:
-
p-Methoxymethamphetamine
- PPP:
-
α-Pyrrolidinopropiophenone
- Prolintane:
-
1-(1-Benzylbutyl)pyrrolidine
- Pyrovalerone (MPVP):
-
4′-Methyl-α-pyrrolidinovalerophenone
- PVP (α-PVP):
-
α-Pyrrolidinovalerophenone
- α-PVT:
-
α-Pyrrolidinopentiothiophenone
References
Kalix P (1992) Cathinone, a natural amphetamine. Pharmacol Toxicol 70:77–86
Zaitsu K, Katagi M, Tatsuno M, Sato T, Tsuchihashi H, Suzuki K (2011) Recently abused β-keto derivatives of 3,4-methylenedioxyphenylalkylamines: a review of their metabolisms and toxicological analysis. Forensic Toxicol 29:73–84
Kelly JP (2011) Cathinone derivatives: a review of their chemistry, pharmacology and toxicology. Drug Test Anal 3:439–453
Zawilska JB, Wojcieszak J (2013) Designer cathinones—an emerging class of novel recreational drugs. Forensic Sci Int 231:42–53
Ammann D, McLaren JM, Gerostamoulos D, Beyer J (2012) Detection and quantification of new designer drugs in human blood: part 2—designer cathinones. J Anal Toxicol 36:381–389
Kikura-Hanajiri R, Uchiyama N, Kawamura M, Goda Y (2013) Changes in the prevalence of synthetic cannabinoids and cathinone derivatives in Japan until early 2012. Forensic Toxicol 31:44–53
Chung H, Choi H, Heo S, Kim E, Lee J (2013) Synthetic cannabinoids abused in South Korea: drug identifications by the National Forensic Service from 2009 to June 2013. Forensic Toxicol. doi:10.1007/s11419-013-0213-6
Westphal F, Junge T, Klein B, Fritschi G, Girreser U (2011) Spectroscopic characterization of 3,4-methylenedioxypyrrolidinobutyrophenone: a new designer drug with α-pyrrolidinophenone structure. Forensic Sci Int 209:126–132
Brandt SD, Sumnall HR, Measham F, Cole J (2010) Analyses of second-generation ‘legal highs’ in the UK: initial findings. Drug Test Anal 2:377–382
De Paoli G, Maskell PD, Pounder DJ (2011) Naphyrone: analytical profile of the new “legal high” substitute for mephedrone. J Forensic Legal Med 18:93
Uchiyama N, Matsuda S, Kawamura M, Kikura-Hanajiri R, Goda Y (2013) Two new-type cannabimimetic quinolinyl carboxylates, QUPIC and QUCHIC, two new cannabimimetic carboxamide derivatives, ADB-FUBINACA and ADBICA, and five synthetic cannabinoids detected with a thiophene derivative α-PVT and an opioid receptor agonist AH-7921 identified in illegal products. Forensic Toxicol 31:223–240
Nencini P, Ahmed AM (1989) Khat consumption: a pharmacological review. Drug Alcohol Depend 23:19–29
Hollister LE, Gillespie HK (1970) A new stimulant, prolintane hydrochloride, compared with dextroamphetamine in fatigued volunteers. J Clin Pharmacol J New Drugs 10:103–109
Gardos G, Cole JO (1971) Evaluation of pyrovalerone in chronically fatigued volunteers. Curr Ther Res Clin Exp 13:631–635
Cozzi NV, Sievert MK, Shulgin AT, Jacob P 3rd, Ruoho AE (1999) Inhibition of plasma membrane monoamine transporters by β-ketoamphetamines. Eur J Pharmacol 381:63–69
Kehr J, Ichinose F, Yoshitake S, Goiny M, Sievertsson T, Nyberg F, Yoshitake T (2011) Mephedrone, compared with MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and 5-HT levels in nucleus accumbens of awake rats. Br J Pharmacol 164:1949–1958
Baumann MH, Ayestas MA, Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV (2012) The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology 37:1192–1203
Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener MC, Liechti ME (2013) Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol 168:458–470
Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, Rothman RB, Goldberg SR, Lupica CR, Sitte HH, Brandt SD, Tella SR, Cozzi NV, Schindler CW (2013) Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology 38:552–562
López-Arnau R, Martínez-Clemente J, Pubill D, Escubedo E, Camarasa J (2012) Comparative neuropharmacology of three psychostimulant cathinone derivatives: butylone, mephedrone and methylone. Br J Pharmacol 167:407–420
Martínez-Clemente J, Escubedo E, Pubill D, Camarasa J (2012) Interaction of mephedrone with dopamine and serotonin targets in rats. Eur Neuropsychopharmacol 22:231–236
Coppola M, Mondola R (2012) 3,4-Methylenedioxypyrovalerone (MDPV): chemistry, pharmacology and toxicology of a new designer drug of abuse marketed online. Toxicol Lett 208:12–15
Dargan PI, Sedefov R, Gallegos A, Wood DM (2011) The pharmacology and toxicology of the synthetic cathinone mephedrone (4-methylmethcathinone). Drug Test Anal 3:454–463
Gustavsson D, Escher C (2009) Mephedrone–Internet drug which seems to have come and stay. Fatal cases in Sweden have drawn attention to previously unknown substance. (in Swedish and English abstract). Lakartidningen 106:2769–2771
Wood DM, Greene SL, Dargan PI (2011) Clinical pattern of toxicity associated with the novel synthetic cathinone mephedrone. Emerg Med J 28:280–282
Borek HA, Holstege CP (2012) Hyperthermia and multiorgan failure after abuse of “bath salts” containing 3,4-methylenedioxypyrovalerone. Ann Emerg Med 60:103–105
Ross EA, Watson M, Goldberger B (2011) “Bath salts” intoxication. N Engl J Med 365:967–968
Murray B, Murphy C, Beuhler M (2012) Death following recreational use of designer drug “bath salts” containing 3,4-methylenedioxypyrovalerone (MDPV). J Med Toxicol 8:69–75
Albers DS, Sonsalla PK (1995) Methamphetamine-induced hyperthermia and dopaminergic neurotoxicity in mice: pharmacological profile of protective and nonprotective agents. J Pharmacol Exp Ther 275:1104–1114
Hall AP, Henry JA (2006) Acute toxic effects of ‘Ecstasy’ (MDMA) and related compounds: overview of pathophysiology and clinical management. Br J Anaesth 96:678–685
Baumann MH, Rothman RB (2009) Chapter 10—Neural and cardiac toxicities associated with 3,4-methylenedioxymethamphetamine (MDMA). In: Hari Shanker S (ed) International review of neurobiology, vol. 88. Academic Press, New York, pp 257–296
Aarde SM, Huang PK, Creehan KM, Dickerson TJ, Taffe MA (2013) The novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: self-administration and locomotor activity in rats. Neuropharmacology 71:130–140
Mugele J, Nañagas KA, Tormoehlen LM (2012) Serotonin syndrome associated with MDPV use: a case report. Ann Emerg Med 60:100–102
Adebamiro A, Perazella MA (2012) Recurrent acute kidney injury following bath salts intoxication. Am J Kidney Dis 59:273–275
Sauer C, Hoffmann K, Schimmel U, Peters FT (2011) Acute poisoning involving the pyrrolidinophenone-type designer drug 4′-methyl-alpha-pyrrolidinohexanophenone (MPHP). Forensic Sci Int 208:e20–e25
Saito T, Namera A, Osawa M, Aoki H, Inokuchi S (2013) SPME–GC–MS analysis of α-pyrrolidinovaleorophenone in blood in a fatal poisoning case. Forensic Toxicol 31:328–332
Eiden C, Mathieu O, Cathala P, Debruyne D, Baccino E, Petit P, Peyriere H (2013) Toxicity and death following recreational use of 2-pyrrolidino valerophenone. Clin Toxicol 51:899–903
Michaelis W, Russel JH, Schindler O (1970) Metabolism of pyrovalerone hydrochloride. J Med Chem 13:497–503
Shin H-S, Shin Y-SO, Lee S, Park B-B (1996) Detection and identification of pyrovalerone and its hydroxylated metabolite in the rat. J Anal Toxicol 20:568–572
Lho D-S, Lee J, Kim S, Park J, Shin H-S (1996) Identification of a pyrovalerone metabolite in the rat by gas chromatography-mass spectrometry and determination of pyrovalerone by gas chromatography-nitrogen-phosphorus detection. J Chromatogr B 687:253–259
Springer D, Peters FT, Fritschi G, Maurer HH (2002) Studies on the metabolism and toxicological detection of the new designer drug 4′-methyl-α-pyrrolidinopropiophenone in urine using gas chromatography-mass spectrometry. J Chromatogr B 773:25–33
Springer D, Peters FT, Fritschi G, Maurer HH (2003) New designer drug 4′-methyl-α-pyrrolidinohexanophenone: studies on its metabolism and toxicological detection in urine using gas chromatography–mass spectrometry. J Chromatogr B 789:79–91
Springer D, Fritschi G, Maurer HH (2003) Metabolism of the new designer drug α-pyrrolidinopropiophenone (PPP) and the toxicological detection of PPP and 4′-methyl-α-pyrrolidinopropiophenone (MPPP) studied in rat urine using gas chromatography-mass spectrometry. J Chromatogr B 796:253–266
Springer D, Fritschi G, Maurer HH (2003) Metabolism and toxicological detection of the new designer drug 4′-methoxy-α-pyrrolidinopropiophenone studied in rat urine using gas chromatography–mass spectrometry. J Chromatogr B 793:331–342
Springer D, Fritschi G, Maurer HH (2003) Metabolism and toxicological detection of the new designer drug 3′,4′-methylenedioxy-α-pyrrolidinopropiophenone studied in urine using gas chromatography–mass spectrometry. J Chromatogr B 793:377–388
Peters FT, Meyer MR, Fritschi G, Maurer HH (2005) Studies on the metabolism and toxicological detection of the new designer drug 4′-methyl-α-pyrrolidinobutyrophenone (MPBP) in rat urine using gas chromatography–mass spectrometry. J Chromatogr B 824:81–91
Sauer C, Peters FT, Haas C, Meyer MR, Fritschi G, Maurer HH (2009) New designer drug α-pyrrolidinovalerophenone (PVP): studies on its metabolism and toxicological detection in rat urine using gas chromatographic/mass spectrometric techniques. J Mass Spectrom 44:952–964
Strano-Rossi S, Cadwallader AB, de la Torre X, Botrè F (2010) Toxicological determination and in vitro metabolism of the designer drug methylenedioxypyrovalerone (MPDV) by gas chromatography/mass spectrometry and liquid chromatography/quadrupole time-of-flight mass spectrometry. Rapid Comm Mass Spectrom 24:2706–2714
Meyer MR, Du P, Schuster F, Maurer HH (2010) Studies on the metabolism of the α-pyrrolidinophenone designer drug methylenedioxy-pyrovalerone (MDPV) in rat and human urine and human liver microsomes using GC–MS and LC–high-resolution MS and its detectability in urine by GC–MS. J Mass Spectrom 45:1426–1442
Tyrkkö E, Pelander A, Ketola R, Ojanperä I (2013) In silico and in vitro metabolism studies support identification of designer drugs in human urine by liquid chromatography/quadrupole-time-of-flight mass spectrometry. Anal Bioanal Chem 405:6697–6709
Namera A, Konuma K, Kawamura M, Saito T, Nakamoto A, Yahata M, Ohta S, Miyazaki S, Shiraishi H, Nagao M (2013) Time-course profile of urinary excretion of intravenously administered α-pyrrolidinovalerophenone and α-pyrrolidinobutiophenone in a human. Forensic Toxicol. doi:10.1007/s11419-013-0203-8
Shima N, Katagi M, Kamata H, Matsuta S, Sasaki K, Kamata T, Nishioka H, Miki A, Tatsuno M, Zaitsu K, Ishii A, Sato T, Tsuchihashi H, Suzuki K (2013) Metabolism of the newly encountered designer drug α-pyrrolidinovalerophenone in humans: identification and quantitation of urinary metabolites. Forensic Toxicol. doi:10.1007/s11419-013-0202-9
Beckett AH, Midha KK (1974) The identification of four metabolic products after incubation of p-methoxyamphetamine with liver preparations of various species. Xenobiotica 4:297–311
Hubbard JW, Midha KK, Cooper JK (1977) The metabolism of p-methoxyamphetamine in dog and monkey. O-Demethylation as a major route. Drug Metab Dispos 5:329–334
Kitchen I, Tremblay J, André J, Dring LG, Idle JR, Smith RL, Williams RT (1979) Interindividual and interspecies variation in the metabolism of the hallucinogen 4-methoxyamphetamine. Xenobiotica 9:397–404
Staack RF, Fehn J, Maurer HH (2003) New designer drug p-methoxymethamphetamine: studies on its metabolism and toxicological detection in urine using gas chromatography–mass spectrometry. J Chromatogr B 789:27–41
Staack RF, Maurer HH (2005) Metabolism of designer drugs of abuse. Curr Drug Metab 6:259–274
Zaitsu K, Katagi M, Kamata T, Kamata H, Shima N, Tsuchihashi H, Hayashi T, Kuroki H, Matoba R (2008) Determination of a newly encountered designer drug “p-methoxyethylamphetamine” and its metabolites in human urine and blood. Forensic Sci Int 177:77–84
Segura M, Ortuño J, Farré M, McLure JA, Pujadas M, Pizarro N, Llebaria A, Joglar J, Roset PN, Segura J, de la Torre R (2001) 3,4-Dihydroxymethamphetamine (HHMA). A major in vivo 3,4-methylenedioxymethamphetamine (MDMA) metabolite in humans. Chem Res Toxicol 14:1203–1208
Kamata HT, Shima N, Zaitsu K, Kamata T, Miki A, Nishikawa M, Katagi M, Tsuchihashi H (2006) Metabolism of the recently encountered designer drug, methylone, in humans and rats. Xenobiotica 36:709–723
Shima N, Katagi M, Kamata H, Zaitsu K, Kamata T, Nishikawa M, Miki A, Tsuchihashi H, Sakuma T, Nemoto N (2008) Urinary excretion of the main metabolites of 3,4-methylenedioxymethamphetamine (MDMA), including the sulfate and glucuronide of 4-hydroxy-3-methoxymethamphetamine (HMMA), in humans and rats. Xenobiotica 38:314–324
Zaitsu K, Katagi M, Kamata HT, Kamata T, Shima N, Miki A, Tsuchihashi H, Mori Y (2009) Determination of the metabolites of the new designer drugs bk-MBDB and bk-MDEA in human urine. Forensic Sci Int 188:131–139
Conflict of interest
There are no financial or other relations that could lead to a conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zaitsu, K., Katagi, M., Tsuchihashi, H. et al. Recently abused synthetic cathinones, α-pyrrolidinophenone derivatives: a review of their pharmacology, acute toxicity, and metabolism. Forensic Toxicol 32, 1–8 (2014). https://doi.org/10.1007/s11419-013-0218-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11419-013-0218-1