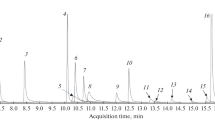
Abstract
Six novel hallucinogens classed as (tetrahydrobenzodifuranyl) aminoalkanes or (benzodifuranyl)aminoalkanes, which are known by the common names of “FLY” and “DragonFLY,” respectively, were synthesized. These compounds were simultaneously analyzed by gas chromatography (GC)-mass spectrometry (MS), liquid chromatography (LC)-MS, and LC-MS-MS. GCMS analysis of their free bases was not satisfactory for both mass spectral and chromatographic measurements, and thus trifluoroacetyl (TFA) derivatization was employed. However, it was found that the usual TFA derivatization procedure using trifluoroacetic anhydride caused dehydrogenation of FLYs to the corresponding DragonFLYs. Therefore, TFA derivatization of FLYs was reinvestigated; the presence of triethylamine could almost inhibit such dehydrogenation. LC separation of the analytes was successfully achieved by using a phenyl-type semimicro column with methanol gradient elution, while 1-(8-bromo-2,3,6,7-tetrahydrobenzo[1,2-b;4,5-b′]difuran-4-yl)-2-methylaminopropane (N-methyl-DOB-FLY) and 1-(8-bromo-2,3,6,7-tetrahydrobenzo[1,2-b;4,5-b′]difuran-4-yl)-2-aminopropane (DOB-FLY) were not separated on an octadecylsilica (ODS)-type column. Specific product ion spectra for all compounds were also obtained using LC-MS-MS, which enabled sensitive and reliable identification.
Similar content being viewed by others
References
Monte AP, Waldman SR, Marona-Lewicka D, Wainscott DB, Nelson DL, Sanders-Bush E, Nichols DE (1997) Dihydrobenzofuran analogues of hallucinogens. 4. Mescaline derivatives. J Med Chem 40:2997–3008
Parker MA, Marona-Lewicka D, Lucaites VL, Nelson DL, Nichols DE (1998) A novel (benzodifuranyl)aminoalkane with extremely potent activity at the 5-HT2A receptor. J Med Chem 41:5148–5149
Chambers JJ, Kurrasch-Orbaugh DM, Parker MA, Nichols DE (2001) Enantiospecific synthesis and pharmacological evaluation of a series of super-potent, conformationally restricted 5-HT2A/2C receptor agonists. J Med Chem 44:1003–1010
Reed EC, Kiddon GS (2007) The characterization of three FLY compounds (2C-B-FLY, 3C-B-FLY, and Bromo-DragonFLY). Microgram J 5:26–33
Anonymous (2007) “Bromo dragonfly” (bromo-benzodifuranyl-isopropylamine) in Ashland, Oregon. Microgram Bull 40:78
Anonymous (2008) “Bromo-Dragonfly” in Queensland, Australia. Microgram Bull 41:16–17
Erowid (2009) The vaults of Erowid. http://www.erowid.org/chemicals/bromo_dragonfly/bromo_dragonfly.shtml. Cited August 2009
Andreasen MF, Telving R, Birkler RID, Schumacher B, Johannsen M (2009) A fatal poisoning involving Bromo-Dragonfly. Forensic Sci Int 183:91–96
Monte AP, Marona-Lewicka D, Parker MA, Wainscott DB, Nelson DL, Nichols DE (1996) Dihydrobenzofuran analogues of hallucinogens. 3. Models of 4-substituted (2,5-dimethoxyp henyl)alkylamine derivatives with rigidified methoxy groups. J Med Chem 39:2953–2961
Pizarro N, de la Torre R, Farré M, Segura J, Llebaria A, Joglar J (2002) Synthesis and capillary electrophoretic analysis of enantiomerically enriched reference standards of MDMA and its main metabolites. Bioorg Med Chem 10:1085–1092
Zaitsu K, Katagi M, Kamata H, Kamata T, Shima N, Miki A, Iwamura T, Tsuchihashi H (2008) Discrimination and identification of the six aromatic positional isomers of trimethoxyamphetamine (TMA) by gas chromatography-mass spectrometry (GC-MS). J Mass Spectrom 43:528–534
Zaitsu K, Katagi M, Kamata HT, Miki A, Tsuchihashi H (2008) Discrimination and identification of regioisomeric β-keto analogues of 3,4-methylenedioxyamphetamines by gas chromatography-mass spectrometry. Forensic Toxicol 26:45–51
Kovats ES (1965) Gas chromatographic characterization of organic substances in the retention index system. Adv Chromatogr 1:229–247
Kanamori T, Iwata Y, Ohmae Y, Inoue H, Kishi T (2001) Analysis of 2,5-dimethoxy-4-alkylthiophenethylamines (2CT-analogs) (in Japanese). Jpn J Sci Technol Iden 5:97–103
McLafferty FW, Turecek F (1993) Interpretation of mass spectra, 4th edn. University Science, Mill Valley, CA
Smith RM, Busch KL (1999) Understanding mass spectra—a basic approach. Wiley, New York
Katagi M, Tsutsumi H, Miki A, Nakajima K, Tsuchihashi H (2002) Analyses of clandestine tablets of amphetamine and their related designer drugs encountered in recent Japan. Jpn J Forensic Toxicol 20:303–319
Zaitsu K, Katagi M, Kamata T, Kamata H, Shima N, Tsuchihashi H, Hayashi T, Kuroki H, Matoba R (2008) Determination of a newly encountered designer drug “pmethoxyethylamphetamine” and its metabolites in human urine and blood. Forensic Sci Int 177:77–84
Zaitsu K, Katagi M, Kamata HT, Kamata T, Shima N, Miki A, Tsuchihashi H, Yasushige M (2009) Determination of the metabolites of the new designer drugs bk-MBDB and bk-MDEA in human urine. Forensic Sci Int 188: 131–139
Kanai K, Takekawa K, Kumamoto T, Ishikawa T, Ohmori T (2008) Simultaneous analysis of six phenethylamine-type designer drugs by TLC, LC-MS, and GC-MS. Forensic Toxicol 26:6–12
Takayama N, Hayakawa K (2005) Amphetamines and their metabolites. In: Suzuki O, Watanabe K (eds) Drugs and poisons in humans: a handbook of practical analysis. Springer, Berlin Heidelberg New York, pp 171–185
Patai S (1974) The chemistry of the quinonoid compounds. Wiley, New York
Kamata HT, Shima N, Zaitsu K, Kamata T, Miki A, Nishikawa M, Katagi M, Tsuchihashi H (2006) Metabolism of the recently encountered designer drug, methylone in humans and rats. Xenobiotica 36:709–723
Kamata HT, Shima N, Zaitsu K, Kamata T, Nishikawa M, Katagi M, Miki A, Tsuchihashi H (2007) Simultaneous analysis of new designer drug, methylone, and its metabolites in urine by gas chromatography-mass spectrometry and liquid chromatography-electrospray ionization mass spectrometry. Jpn J Forensic Sci Technol 12:97–106
Shima N, Katagi M, Kamata H, Zaitsu K, Kamata T, Miki A, Tsuchihashi H, Sakuma T, Nemoto N (2008) Conjugates of p-hydroxymethamphetamine and 4-hydroxy-3-methoxymethamphetamine in blood obtained from methamphetamine and 3,4-methylendioxymethamphetamine users: analysis by LC-MS-MS. Forensic Toxicol 26:58–65
Maurer HH (1998) Liquid chromatography-mass spectrometry in forensic and clinical toxicology. J Chromatogr B 713: 3–25
Smith ML, Vorce SP, Holler JM, Shimomura E, Magluilo J, Jacobs AJ, Huestis MA (2007) Modern instrumental methods in forensic toxicology. J Anal Toxicol 31:237–253
Decaestecjer T, De Letter E, Clauwaert K, Bouche MP, Lambert W, Van Bocxlaer J, Piette M, Van den Eeckhout E, Van Peteghem C, De Leenheer A (2001) Fatal 4-MTA intoxication: development of a liquid chromatographic-tandem mass spectrometric assay for multiple matrices. J Anal Toxicol 25:705–710
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zaitsu, K., Katagi, M., Kamata, H. et al. Simultaneous analysis of six novel hallucinogenic (tetrahydrobenzodifuranyl)aminoalkanes (FLYs) and (benzodifuranyl)aminoalkanes (DragonFLYs) by GC-MS, LC-MS, and LC-MS-MS. Forensic Toxicol 28, 9–18 (2010). https://doi.org/10.1007/s11419-009-0083-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11419-009-0083-0