Abstract
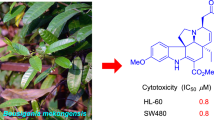
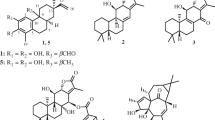
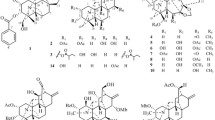
A total of five new mexicanolides (1–5), namely alliaxylines A–E, together with two known limonoids 6 and 7, were isolated and identified from Dysoxylum alliaceum (Blume) Blume ex. A.Juss. (Meliaceae). The structures of these compounds were elucidated based on extensive spectroscopic analyses, including HR-ESI–MS, UV, IR, 1D, and 2D NMR, as well as theoretical stimulation of NMR shifts with the DP4 + algorithm. Consequently, this study aimed to examine cytotoxic activities of these compounds against MCF-7 and A549 cell lines. The results implied that compound 2 was the most potent against the two tested cells, with IC50 values of 34.95 ± 0.21 and 44.39 ± 1.03 µM.
Graphical Abstract




Similar content being viewed by others
References
Luo J, Sun Y, Li Q, Kong L (2022) Research progress of meliaceous limonoids from 2011 to 2021. Nat Prod Rep 39:1325–1365. https://doi.org/10.1039/D2NP00015F
Riyadi SA, Naini AA, Supratman U (2023) Sesquiterpenoids from meliaceae family and their biological activities. Molecules 28:4874. https://doi.org/10.3390/molecules28124874
Nugroho AE, Wong CP, Hirasawa Y, Kaneda T, Tougan T, Horii T, Morita H (2023) Antimalarial ceramicines Q-T from Chisocheton ceramicus. J Nat Med 77:596–603. https://doi.org/10.1007/s11418-023-01706-w
Tringali C (2000) Bioactive compounds from natural sources: isolation characterization and biological properties. Taylor & Francis, Abingdon
Naini AA, Mayanti T, Maharani R, Fajriah S, Kabayama K, Shimoyama A, Manabe Y, Supratman U (2023) Dysoticans F-H: three unprecedented dimeric cadinanes from Dysoxylum parasiticum (Osbeck) Kosterm. stem bark. RSC Adv 13:9370–9376. https://doi.org/10.1039/D3RA01085F
Naini AA, Mayanti T, Harneti D, Maharani R, Farabi K, Herlina T, Supratman U, Chakthong S (2023) Sesquiterpenoids and sesquiterpenoid dimers from the stem bark of Dysoxylum parasiticum (osbeck) kosterm. Phytochemistry 205:113477. https://doi.org/10.1016/j.phytochem.2022.113477
Nagakura Y, Yamanaka R, Hirasawa Y, Hosoya T, Rahman A, Kusumawati I, Morita H (2010) Gaudichaudysolin A, a new limonoid from the bark of Dysoxylum gaudichaudianum. Heterocycles 80:1471–1477. https://doi.org/10.3987/COM-09-S(S)106
Chen JL, Kernan MR, Jolad SD, Stoddart CA, Bogan M, Cooper R (2007) Dysoxylins A−D, Tetranortriterpenoids with Potent Anti-RSV Activity from Dysoxylum gaudichaudianum. J Nat Prod 70:312–315. https://doi.org/10.1021/np060398y
Luo XD, Wu SH, Wu DG, Ma YB, Qi SH (2002) Novel antifeeding limonoids from Dysoxylum hainanense. Tetrahedron 58:7797–7804. https://doi.org/10.1016/S0040-4020(02)00944-4
Han ML, Zhao JX, Liu HC, Ni G, Ding J, Yang SP, Yue JM (2015) Limonoids and Triterpenoids from Dysoxylum mollissimum var. glaberrimum. J Nat Prod 78:754–761. https://doi.org/10.1021/np500967k
Lakshmi V, Pandey K, Agarwal SK (2009) Bioactivity of the compounds in genus Dysoxylum. Acta Ecol Sin 29:30–44. https://doi.org/10.1016/j.chnaes.2009.04.005
Suwardi AB, Navia ZI, Harmawan T, Seprianto S, Syamsuardi S, Mukhtar E (2022) Diversity of wild edible fruit plant species and their threatened status in the Aceh Province, Indonesia. Biodiversitas 23:1310–1318. https://doi.org/10.13057/biodiv/d230315
Nishizawa M, Inoue A, Sastrapradja S, Hayashi Y (1983) (+)-8-Hydroxycalamenene: a fish-poison principle of Dysoxylum acutangulum and D. Alliaceum Phytochem 22:2083–2085. https://doi.org/10.1016/0031-9422(83)80052-1
Nishizawa M, Yamada H, Sastrapradja S, Hayashi Y (1985) Structure and synthesis of bicalamenene. Tetrahedron Lett 26:1535–1536. https://doi.org/10.1016/S0040-4039(00)98545-9
Lin BD, Yuan T, Zhang CR, Dong L, Zhang B, Wu Y, Yue JM (2009) Structurally diverse limonoids from the fruits of Swietenia mahagoni. J Nat Prod 72:2084–2090. https://doi.org/10.1021/np900522h
Zhang JC, Liao Q, Shen L, Wu J (2020) Twenty-five limonoids from the Hainan mangrove Xylocarpus granatum. Bioorganic Chem 100:103903. https://doi.org/10.1016/j.bioorg.2020.103903
Pudhom K, Sommit D, Nuclear P, Ngamrojanavanich N, Petsom A (2010) Moluccensins H− J, 30-Ketophragmalin Limonoids from Xylocarpus moluccensis. J Nat Prod 73:263–266. https://doi.org/10.1021/np900583h
Hofer M, Greger H, Mereiter K (2009) 6α-Acetoxygedunin. Acta Crystallographica Sect E: Struct Rep Online 65:1942–1943
Kipassa NT, Iwagawa T, Okamura H, Doe M, Morimoto Y, Nakatani M (2008) Limonoids from the stem bark of Cedrela odorata. Phytochemistry 69:1782–1787. https://doi.org/10.1016/j.phytochem.2007.12.015
Deng Z, Liu S, Cui S, Zhou L, Yao Q (2012) Cytotoxic activity of five limonoids from Meliae cortex and their structure-activity relationship. Chin J Nat Med 10:238–240. https://doi.org/10.3724/SP.J.1009.2012.00238
Grimblat N, Zanardi MM, Sarotti AM (2015) Beyond DP4+ An improved probability for the stereochemical assignment of isomeric compounds using quantum chemical calculations of NMR shifts. J Org Chem 80:12526–12534. https://doi.org/10.1021/acs.joc.5b02396
Acknowledgements
The authors would like to thank the facilities of Universitas Padjadjaran, Centre for Higher Education Funding (BPPT) Ministry of Education, Culture, Research and Technology, also the Indonesia Endowment Fund for Education (LPDP).
Funding
This work was partly supported by Academic Leadership Grant No 1959/UN6.3.1/PT.00/2023 awarded to Unang Supratman, by Centre for Higher Education Funding (BPPT) Ministry of Education, Culture, Research and Technology, and also by the Indonesia Endowment Fund for Education (LPDP).
Author information
Authors and Affiliations
Contributions
US, TM, RL, and SJ assisted in conducting the experiments, performed the spectral analysis, and wrote the manuscript. SAR, AAN, SF, and MNA designed and conducted all experiments, as well as wrote the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Riyadi, S.A., Naini, A.A., Mayanti, T. et al. Alliaxylines A–E: five new mexicanolides from the stem barks of Dysoxylum alliaceum (Blume) Blume ex A.Juss. J Nat Med (2024). https://doi.org/10.1007/s11418-024-01794-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11418-024-01794-2