Abstract
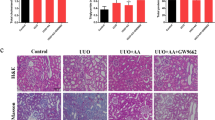
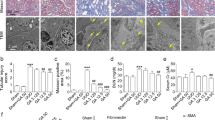
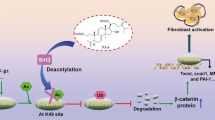
Tubulointerstitial fibrosis is a common pathological change in end-stage renal disease. However, limited treatment methods are developed, and unexplained potential mechanisms of renal diseases are urgent problems to be solved. In the present research, we first elucidated the role of podocarpusflavone (POD), a biflavone compound, in unilateral ureteral obstruction (UUO) in rodent model which is characterized by inflammation and fibrosis. The changes in histology and immunohistochemistry were observed that POD exerted renoprotective effects by retarding the infiltration of macrophage and aberrant deposition of ɑ-SMA, Col1a1, and fibronectin. Consistent with in vivo assay, POD treatment also ameliorated the process of fibrosis in TGF-β1-stimulated renal tubular epithelial cells and inflammation in LPS-induced RAW264.7 cells in vitro. In terms of mechanism, our results showed that treatment with POD inhibited the aggravated activation of Fyn in the UUO group, and weakened the level of phosphorylation of Stat3 which indicated that POD may alleviate the process of fibrosis by the Fyn/Stat3 signaling pathway. Furthermore, the gain of function assay by lentivirus-mediated exogenous forced expression of Fyn abrogated the therapeutic effect of the POD on renal fibrosis and inflammation. Collectively, it can be concluded that POD exerted a protective effect on renal fibrosis by mediating Fyn/Stat3 signaling pathway.






Similar content being viewed by others
Data availability
No additional data are available.
References
Gela D, Mengistu D (2018) Self-management and associated factors among patients with end-stage renal disease undergoing hemodialysis at health facilities in Addis Ababa, Ethiopia. Int J Nephrol Renovasc Dis 11:329–336. https://doi.org/10.2147/IJNRD.S184671
Cheng Z, Liu L, Wang Z et al (2018) Hypoxia activates Src and promotes endocytosis which decreases MMP-2 activity and aggravates renal interstitial fibrosis. Int J Mol Sci 19:581. https://doi.org/10.3390/ijms19020581
Liu B, Ding F, Hu D et al (2018) Human umbilical cord mesenchymal stem cell conditioned medium attenuates renal fibrosis by reducing inflammation and epithelial-to-mesenchymal transition via the TLR4/NF-κB signaling pathway in vivo and in vitro. Stem Cell Res Ther 9:7. https://doi.org/10.1186/s13287-017-0760-6
Shang P, Liu T, Liu W et al (2017) Telmisartan improves vascular remodeling through ameliorating prooxidant and profibrotic mechanisms in hypertension via the involvement of transforming growth factor-β1. Mol Med Rep 16:4537–4544. https://doi.org/10.3892/mmr.2017.7177
Alvandi Z, Opas M (2020) c-Src kinase inhibits osteogenic differentiation via enhancing STAT1 stability. PLoS ONE 15:e0241646. https://doi.org/10.1371/journal.pone.0241646
Han Z-H, Wang F, Wang F-L et al (2018) Regulation of transforming growth factor β-mediated epithelial-mesenchymal transition of lens epithelial cells by c-Src kinase under high glucose conditions. Exp Ther Med 16:1520–1528. https://doi.org/10.3892/etm.2018.6348
Wei C, Banu K, Garzon F et al (2018) SHROOM3-FYN interaction regulates nephrin phosphorylation and affects albuminuria in allografts. J Am Soc Nephrol 29:2641–2657. https://doi.org/10.1681/ASN.2018060573
Seo H-Y, Jeon J-H, Jung Y-A et al (2016) Fyn deficiency attenuates renal fibrosis by inhibition of phospho-STAT3. Kidney Int 90:1285–1297. https://doi.org/10.1016/j.kint.2016.06.038
Yu J, Zhou Z, Wei Z et al (2020) FYN promotes gastric cancer metastasis by activating STAT3-mediated epithelial–mesenchymal transition. Transl Oncol 13:100841. https://doi.org/10.1016/j.tranon.2020.100841
Varga J, Pasche B (2009) Transforming growth factor-ß as a therapeutic target in systemic sclerosis. Nat Rev Rheumatol 5:200–206. https://doi.org/10.1038/nrrheum.2009.26
Wang G, Yao S, Zhang X-X, Song H (2015) Rapid screening and structural characterization of antioxidants from the extract of Selaginella doederleinii Hieron with DPPH-UPLC-Q-TOF/MS method. Int J Anal Chem 2015:849769. https://doi.org/10.1155/2015/849769
Trang DT, Huyen LT, Nhiem NX et al (2016) Tirucallane glycoside from the leaves of Antidesma bunius and inhibitory NO production in BV2 cells and RAW264.7 macrophages. Nat Prod Commun 11:935–937
Meng H, Pang Y, Liu G et al (2020) Podocarpusflavone A inhibits cell growth of skin cutaneous melanoma by suppressing STAT3 signaling. J Dermatol Sci 100:201–208. https://doi.org/10.1016/j.jdermsci.2020.10.008
Chung KW, Jeong HO, Lee B et al (2017) Involvement of NF-κBIZ and related cytokines in age-associated renal fibrosis. Oncotarget 8:7315–7327. https://doi.org/10.18632/oncotarget.14614
Li J, Yang J, Zhu B et al (2022) Tectorigenin protects against unilateral ureteral obstruction by inhibiting Smad3-mediated ferroptosis and fibrosis. Phytother Res 36:475–487. https://doi.org/10.1002/ptr.7353
Cui L, Li C, Shang Y et al (2021) Chaihu Guizhi Ganjiang decoction ameliorates pancreatic fibrosis via JNK/mTOR signaling pathway. Front Pharmacol 12:679557. https://doi.org/10.3389/fphar.2021.679557
Dorotea D, Jiang S, Pak ES et al (2022) Pan-Src kinase inhibitor treatment attenuates diabetic kidney injury via inhibition of Fyn kinase-mediated endoplasmic reticulum stress. Exp Mol Med 54:1086–1097. https://doi.org/10.1038/s12276-022-00810-3
Afroz R, Kumarapperuma H, Nguyen QVN et al (2022) Lipopolysaccharide acting via toll-like receptor 4 transactivates the TGF-β receptor in vascular smooth muscle cells. Cell Mol Life Sci 79:121. https://doi.org/10.1007/s00018-022-04159-8
Taniguchi K, Xia L, Goldberg HJ et al (2013) Inhibition of Src kinase blocks high glucose-induced EGFR transactivation and collagen synthesis in mesangial cells and prevents diabetic nephropathy in mice. Diabetes 62:3874–3886. https://doi.org/10.2337/db12-1010
Yang R, Xu X, Li H et al (2017) p53 induces miR199a-3p to suppress SOCS7 for STAT3 activation and renal fibrosis in UUO. Sci Rep 7:43409. https://doi.org/10.1038/srep43409
Feng X, Lu T-C, Chuang PY et al (2009) Reduction of Stat3 activity attenuates HIV-induced kidney injury. J Am Soc Nephrol 20:2138–2146. https://doi.org/10.1681/ASN.2008080879
Xu Z, Xu Y, Hao Y et al (2017) A novel STAT3 inhibitor negatively modulates platelet activation and aggregation. Acta Pharmacol Sin 38:651–659. https://doi.org/10.1038/aps.2016.155
Xu Q, Liu Y, Pan H et al (2019) Aberrant expression of miR-125a-3p promotes fibroblast activation via Fyn/STAT3 pathway during silica-induced pulmonary fibrosis. Toxicology 414:57–67. https://doi.org/10.1016/j.tox.2019.01.007
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 82104665); the Science and Technology Department of Sichuan Province (No. 2022YFS0621, No. 2023NSFSC1763); the Luzhou-Southwest Medical University Science and Technology Strategic Cooperation Project (No. 2021LZXNYD-P04, No. 2021LZXNYD-D13); the Funding Program of Southwest Medical University (No. 2021ZKZD022); the Innovation Team Project of Affiliated Traditional Chinese Medicine Hospital of Southwest Medical University (No. 2022-CXTD-03).
Author information
Authors and Affiliations
Contributions
BZ and JL substantially contributed to the conception and design of the study. BZ, RH, YN, HG and XL acquired, analyzed and interpreted the data. BZ drafted the article and critically revised it for important intellectual content. JL and LW agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of the work are appropriately investigated and resolved.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11418_2023_1685_MOESM1_ESM.tif
Supplementary file1 The molecular docking between POD and members of the Src family of kinases. A–E. The interaction between POD and Src (−4.2 kcal/mol) (A), Yes (−5.62 kcal/mol) (B), Lyn (−4.89 kcal/mol) (C), FGR (−4.41 kcal/mol) (D) and Lck (−5.35 kcal/mol) (E).(TIF 28380 KB)
11418_2023_1685_MOESM2_ESM.tif
Supplementary file2 Supplementary Fig. 2 POD reduced inflammation in vitro through a Fyn-dependent mechanism. A–D. The mRNA level of Fyn (A), IL-1β (B), IL-6 (C) and TNF-ɑ (D) in all groups of RAW264.7 cells. F (4, 25)=102.6, F (4, 25)=94.81, F (4, 25)=136.1, F (4, 25)=126.5. ***P < 0.001 vs. the Control group, ###P < 0.001 vs. the LPS group, @@@P < 0.001 vs. the LPS group, n.s. means no significant difference vs the LPS+Fyn-OE group. (TIF 6557 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, B., Han, R., Ni, Y. et al. Podocarpusflavone alleviated renal fibrosis in obstructive nephropathy by inhibiting Fyn/Stat3 signaling pathway. J Nat Med 77, 464–475 (2023). https://doi.org/10.1007/s11418-023-01685-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-023-01685-y