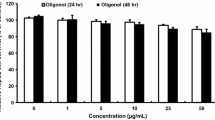
Abstract
The predominant feature of type 2 diabetes is insulin resistance. Identifying a drug able to reduce insulin resistance is an urgent requirement. ent-3α-Formylabieta-8(14),13(15)-dien-16,12β-olide had been identified as a new diterpene derivative which showed anticancer activity. This study explores the hypoglycemic effect of ent-3α-formylabieta-8(14),13(15)-dien-16,12β-olide and studied its mechanism. The insulin response of HepG2 cells following ent-3α-formylabieta-8(14),13(15)-dien-16,12β-olide treatment, as a model for liver cancer cells, was assessed. The results demonstrated that hyperglycemia resulted in a significant increase in the levels of insulin receptor substrate-1 (IRS-1) serine phosphorylation and decrease in Akt phosphorylation. High glucose also inhibited the phosphorylation of insulin-dependent GSK3β. ent-3α-Formylabieta-8(14),13(15)-dien-16,12β-olide treatment improved the effect of insulin on the phosphorylation of IRS-1 Ser307. In addition, this study demonstrated that the effect of ent-3α-formylabieta-8(14),13(15)-dien-16,12β-olide was dependent on the activation of AMP-activated protein kinase. Collectively, experimental data indicated an association between insulin resistance and hyperglycemia in HepG2 cells, and that ent-3α-formylabieta-8(14),13(15)-dien-16,12β-olide reduces IRS-1 Ser307 phosphorylation via activating AMPK, thereby decreasing the insulin signaling blockade.




Similar content being viewed by others
References
Qi X, Li L, Yang G, Liu J, Li K, Tang Y, Liou H, Boden G (2007) Circulating obestatin levels in normal subjects and in patients with impaired glucose regulation and type 2 diabetes mellitus. Clin Endocrinol 66:593–597
Earle KE, Archer AG, Baillie JE (1989) Circulating and excreted levels of chromium after an oral glucose challenge: influence of body mass index, hypoglycemic drugs, and presence and absence of diabetes mellitus. Am J Clin Nutr 49:685–689
Del Prato S (2009) Role of glucotoxicity and lipotoxicity in the pathophysiology of Type 2 diabetes mellitus and emerging treatment strategies. Diabet Med J Br Diabet Assoc 26:1185–1192
Bedard K, Strecko J, Theriault K, Bedard J, Veyrat-Durebex C, Gaudreau P (2008) Effects of a high-glucose environment on the pituitary growth hormone-releasing hormone receptor: type 1 diabetes compared with in vitro glucotoxicity. Am J Physiol Endocrinol Metab 294:E740–751
Lindmark S, Buren J, Eriksson JW (2006) Insulin resistance, endocrine function and adipokines in type 2 diabetes patients at different glycaemic levels: potential impact for glucotoxicity in vivo. Clin Endocrinol 65:301–309
Ahmed F, Waslien C, Al-Sumaie MA, Prakash P, Allafi A (2013) Trends and risk factors of hyperglycemia and diabetes among Kuwaiti adults: National Nutrition Surveillance Data from 2002 to 2009. BMC Public Health 13:103
Tiano JP, Delghingaro-Augusto V, Le May C, Liu S, Kaw MK, Khuder SS, Latour MG, Bhatt SA, Korach KS, Najjar SM, Prentki M, Mauvais-Jarvis F (2011) Estrogen receptor activation reduces lipid synthesis in pancreatic islets and prevents beta cell failure in rodent models of type 2 diabetes. J Clin Investig 121:3331–3342
Kanat M, Serin E, Tunckale A, Yildiz O, Sahin S, Bolayirli M, Arinc H, Dirican A, Karagoz Y, Altuntas Y, Celebi H, Oguz A (2009) A multi-center, open label, crossover designed prospective study evaluating the effects of lipid lowering treatment on steroid synthesis in patients with Type 2 diabetes (MODEST Study). J Endocrinol Invest 32:852–856
Jiang F, Li S, Pan L, Jia C (2015) Association of the G1057D polymorphism in insulin receptor substrate 2 gene with type 2 diabetes mellitus: a meta-analysis. J Diabet Complicat 29:731–736
Xu J, Lin S, Yin J (2015) Effect of gastric bypass operation on expressions of adipic insulin receptor and insulin receptor substrate-1 in rats with type 2 diabetes mellitus. Zhonghua Wei Chang Wai Ke Za Zhi 18:65–68
Zhang Y, Sun CM, Hu XQ, Zhao Y (2014) Relationship between melatonin receptor 1B and insulin receptor substrate 1 polymorphisms with gestational diabetes mellitus: a systematic review and meta-analysis. Sci Rep 4:6113
Oliveira JM, Rebuffat SA, Gasa R, Gomis R (2014) Targeting type 2 diabetes: lessons from a knockout model of insulin receptor substrate 2. Can J Physiol Pharm 92:613–620
De Blasio MJ, Huynh K, Qin C, Rosli S, Kiriazis H, Ayer A, Cemerlang N, Stocker R, Du XJ, McMullen JR, Ritchie RH (2015) Therapeutic targeting of oxidative stress with coenzyme Q10 counteracts exaggerated diabetic cardiomyopathy in a mouse model of diabetes with diminished PI3K(p110alpha) signaling. Free Radical Biol Med 87:137–147
Qi Z, Xu Y, Liang Z, Li S, Wang J, Wei Y, Dong B (2015) Baicalein alters PI3K/Akt/GSK3beta signaling pathway in rats with diabetes-associated cognitive deficits. Int J Clin Exp Med 8:1993–2000
Andrade Ferreira I, Akkerman JW (2005) IRS-1 and vascular complications in diabetes mellitus. Vitam Horm 70:25–67
Aytug S, Reich D, Sapiro LE, Bernstein D, Begum N (2003) Impaired IRS-1/PI3-kinase signaling in patients with HCV: a mechanism for increased prevalence of type 2 diabetes. Hepatology 38:1384–1392
Gelaleti RB, Damasceno DC, Salvadori DM, Marcondes JP, Lima PH, Morceli G, Calderon IM, Rudge MV (2015) IRS-1 gene polymorphism and DNA damage in pregnant women with diabetes or mild gestational hyperglycemia. Diabetol Metab Syndr 7:30
Alharbi KK, Khan IA, Abotalib Z, Al-Hakeem MM (2014) Insulin receptor substrate-1 (IRS-1) Gly927Arg: correlation with gestational diabetes mellitus in Saudi women. Biomed Res Int 2014:146495
Fatchiyah F, Christian N, Soeatmadji D (2013) Reducing IRS-1 activation cause mutation of tyrosine kinase domain hINSR gene on type-2 diabetes mellitus patients. Bioinformation 9:853–857
Liu H, Ou S, Xiao X, Zhu Y, Zhou S (2015) Diabetes worsens ischemia-reperfusion brain injury in rats through GSK-3beta. AM J Med Sci 350:204–211
Liu W, Hao J, Zhu L, Li F, Liu Q, Liu S, Zhao S, Li H, Duan H (2013) Phospho-GSK-3beta is involved in the high-glucose-mediated lipid deposition in renal tubular cells in diabetes. Int J Biochem Cell Biol 45:2066–2075
Kim YK, Lee GS, Jung EM, Hyun SH, Hwang WS, Jeung EB (2012) Generation of fibroblasts overexpressing liver-specific PEPCK in a miniature pig model of human type 2 diabetes mellitus. Mol Med Rep 6:45–50
Samuel VT, Beddow SA, Iwasaki T, Zhang XM, Chu X, Still CD, Gerhard GS, Shulman GI (2009) Fasting hyperglycemia is not associated with increased expression of PEPCK or G6Pc in patients with Type 2 Diabetes. P Natl Acad Sci USA 106:12121–12126
Cadoudal T, Fouque F, Benelli C, Forest C (2008) Glyceroneogenesis and PEPCK-C: pharmacological targets in type 2 diabetes. Med Sci 24:407–413
Ropelle ER, Pauli JR, Fernandes MF, Rocco SA et al (2016) Expression of concern. A central role for neuronal AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) in high-protein diet-induced weight loss. Diabetes 65:1122–1123
Wall CE, Yu RT, Atkins AR, Downes M, Evans RM (2016) Nuclear receptors and AMPK: can exercise mimetics cure diabetes? J Mol Endocrinol 57:R49–58
Kjobsted R, Pedersen AJ, Hingst JR, Sabaratnam R, Birk JB, Kristensen JM, Hojlund K, Wojtaszewski JF (2016) Intact regulation of the AMPK signaling network in response to exercise and insulin in skeletal muscle of male patients with type 2 diabetes: illumination of AMPK activation in recovery from exercise. Diabetes 65:1219–1230
Ding AW, Zhang L (2008) Progress on research of the Euphorbia L. Chin J Tradit Chin Med 26(11):2433–2435
Shi HM, Williams ID, Sung HH, Zhu HX, Ip NY, Min ZD (2005) Cytotoxic diterpenoids from the roots of Euphorbia ebracteolata. Planta Med 71(04):349–354
Madureira AM, Gyemant N, Ascenso JR, Abreu PM, Molnar J, Ferreira MJU (2006) Euphoportlandols A and B, tetracylic diterpene polyesters from Euphorbia portlandica and their anti-MDR effects in cancer cells. J Nat Prod 69(6):950–953
Sudhakar M, Rao CV, Rao PM, Raju DB, Venkateswarlu Y (2006) Antimicrobial activity of Caesalpinia pulcherrima, Euphorbia hirta and Asystasia gangeticum. Fitoterapia 77(5):378–380
Kong LY, Li Y, Wu XL, Min ZD (2002) Cytotoxic diterpenoids from Euphorbia pekinensis. Planta Med 68(3):249–252
Zhao M, Wu S, Li J, Tang WX, Wang JI, Zhang SJ (2014) Chemical constituents from Euphorbia lunulata. China J Chin Mater Med 39(12):2289–2294
Zhang WJ, Weng LJ, Yi LT, Geng D (2016) Chemical constituent in whole herb of Euphorbia lunula. Chin Tradit Herbal Drugs 47(4):554–558
Liu C, Liao ZX, Liu SJ, Qu YB, Wang HS (2014) Two new diterpene derivatives from Euphorbia lunulata Bge and their anti-proliferative activities. Fitoterapia 96:33–38
Long C, Ock KM, Hee SJ, Soon KI, Ye KN et al (2012) Abietane diterpenoids of Rosmarinus officinalis and their diacylglycerol acyltransferase-inhibitory activity. Food Chem 132(4):1775–1780
Bajpai VK, Park YH, Na MK, Kang SC (2015) α-Glucosidase and tyrosinase inhibitory effects of an abietane type diterpenoid taxoquinone from Metasequoia glyptostroboides. BMC Complement Altern Med 15:1–6
Ninon Etsassala GER, Waryo TT, Iwuoha EI, Badmus JA, Marnewick JL, Cupido CN, Hussein AA (2019) Alpha-glucosidase and alpha-amylase inhibitory activities of novel abietane diterpenes from Salvia africana-lutea. Antioxidants 8(10):421
Qu YB, Liao ZX, Liu C, Wang XZ, Zhang J (2018) EFLDO induces apoptosis in hepatic cancer cells by caspase activation in vitro and suppresses tumor growth in vivo. Biomed Pharmacother 100:407–416
Liang L, Guo WH, Esquiliano DR, Asai M, Rodriguez S, Giraud J, Kushner JA, White MF, Lopez MF (2010) Insulin-like growth factor 2 and the insulin receptor, but not insulin, regulate fetal hepatic glycogen synthesis. Endocrinology 151:741–747
Acknowledgements
This work was supported by the National Natural Science Foundation of China (41976109), the Qinghai key R & D and transformation project (Qinghai science and Technology Department) (Grant no. 2017-NK-C25). The Open Project of Qinghai Key Laboratory of Qinghai-Tibet Plateau Biological Resources (Grant no. 2017-ZJ-Y10). The Priority Academic Program Development of Jiangsu Higher Education Institutions (Grant no. 1107047002). Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources (Guangxi Normal University) (Grant no. CMEMR2016-B06).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hou, P., Qu, Y., Liao, Z. et al. A diterpene derivative enhanced insulin signaling induced by high glucose level in HepG2 cells. J Nat Med 74, 434–440 (2020). https://doi.org/10.1007/s11418-019-01384-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-019-01384-7