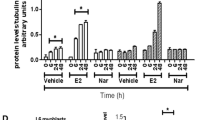
Abstract
Diarylheptanoid, 1-(4-hydroxyphenyl)-7-phenyl(6E)-6-hepten-3-one (HPPH), has been reported to enhance myoblast differentiation via estrogen receptor (ER). However, the underlying signaling pathway promising this action remains unknown. The present study thus aimed to investigate the signaling pathway of HPPH that enhances myoblast differentiation. Confluence C2C12 myoblasts were induced to differentiate in the absence or presence of HPPH (10 nM). Differentiation markers (myosin heavy chain (MHC) and myogenin) and other signaling molecules implicated in myogenic differentiation were analyzed by immunostaining and western blotting methods. To identify the location of ER and the signaling molecules, specific inhibitors were applied targeting these molecules. Nuclear factor-κB (NF-κB) DNA binding activity was measured using the electrophoresis mobility shift assay. The results showed that HPPH enhanced myoblast differentiation by increasing MHC and myogenin levels, number, and size as well as the fusion index of myotubes. These actions occurred via membrane ER. Several MAPK proteins were activated at the early stage of differentiation. However, only Akt and p38 MAPK, but not ERK, were implicated in these effects. The underlying signaling molecules of Akt to enhance myogenic differentiation by HPPH, at least in part, were mTOR/P70S6K and GSK-3β. On the other hand, the downstream signaling molecule of p38 MAPK was NF-κB. Our results suggested that HPPH enhanced myogenic differentiation by binding with membrane ER, which in turn recruited multiple axes including Akt-mTOR-P70S6K, Akt-GSK-3β, and p38 MAPK-NF-κB.








Similar content being viewed by others
Abbreviations
- HPPH:
-
1-(4-Hydroxyphenyl)-7-phenyl(6E)-6-hepten-3-one
- ER:
-
Estrogen receptor
- MHC:
-
Myosin heavy chain
- NF-κB:
-
Nuclear factor kappa B
- EMSA:
-
Electrophoresis mobility shift assay
- BCA:
-
Bicinchoninic acid
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- E2:
-
Estrogen
- MAPK:
-
Mitogen-activated protein kinase
- mTOR:
-
Mammalian target of rapamycin
- P70S6K:
-
70 kDa ribosomal protein S6 kinase
- GSK-3β:
-
Glycogen synthase kinase 3 beta
References
Nagata Y, Partridge TA, Matsuda R, Zammit PS (2006) Entry of muscle satellite cells into the cell cycle requires sphingolipid signaling. J Cell Biol 174:245–253
Wozniak AC, Anderson JE (2007) Nitric oxide-dependence of satellite stem cell activation and quiescence on normal skeletal muscle fibers. Dev Dyn 236:240–250
Sheehan SM, Tatsumi R, Temm-Grove CJ, Allen RE (2000) HGF is an autocrine growth factor for skeletal muscle satellite cells in vitro. Muscle Nerve 23:239–245
Piñol-Jurado P, Gallardo E, de Luna N, Suárez-Calvet X, Sánchez-Riera C, Fernández-Simón E, Gomis C, Illa I, Díaz-Manera J (2017) Platelet-derived growth factor BB influences muscle regeneration in Duchenne muscle dystrophy. Am J Pathol 187:1814–1827
Ye F, Mathur S, Liu M, Borst SE, Walter GA, Sweeney HL, Vandenborne K (2013) Overexpression of insulin-like growth factor-1 attenuates skeletal muscle damage and accelerates muscle regeneration and functional recovery after disuse. Exp Physiol 98:1038–1052
Thomas A, Bunyan K, Tiidus PM (2010) Oestrogen receptor-alpha activation augments post-exercise myoblast proliferation. Acta Physiol 198:81–89
Velders M, Schleipen B, Fritzemeier KH, Zierau O, Diel P (2012) Selective estrogen receptor-beta activation stimulates skeletal muscle growth and regeneration. FASEB J 26:1909–1920
Milanesi L, de Boland AR, Boland R (2008) Expression and localization of estrogen receptor alpha in the C2C12 murine skeletal muscle cell line. J Cell Biochem 104:1254–1273
Milanesi L, Vasconsuelo A, de Boland AR, Boland R (2009) Expression and subcellular distribution of native estrogen receptor beta in murine C2C12 cells and skeletal muscle tissue. Steroids 74:489–497
Galluzzo P, Rastelli C, Bulzomi P, Acconcia F, Pallottini V, Marino M (2009) 17beta-estradiol regulates the first steps of skeletal muscle cell differentiation via ER-alpha mediated signals. Am J Physiol Cell Physiol 297:1249–1262
Suksamrarn A, Ponglikitmongkol M, Wongkrajang K, Chindaduang A, Kittidanairak S, Jankam A, Yingyongnarongkul BE, Kittipanumat N, Chokchaisiri R, Khetkam P, Piyachaturawat P (2008) Diarylheptanoids, new phytoestrogens from the rhizomes of Curcuma comosa: isolation, chemical modification and estrogenic activity evaluation. Bioorg Med Chem 16:6891–6902
Thaloor D, Miller KJ, Gephart J, Mitchell PO, Pavlath GK (1999) Systemic administration of the NF-kappaB inhibitor curcumin stimulates muscle regeneration after traumatic injury. Am J Physiol 277(2 Pt 1):320–329
Tipbunjong C, Kitiyanant Y, Chaturapanich G, Sornkaew N, Suksamrarn A, Kitiyanant N, Esser KA, Pholpramool C (2017) Natural diarylheptanoid compounds from Curcuma comosa Roxb. promote differentiation of mouse myoblasts C2C12 cells selectively via ER alpha receptors. Med Chem Res 26:274
Papini N, Anastasia L, Tringali C, Dileo L, Carubelli I, Sampaolesi M, Monti E, Tettamanti G, Venerando B (2012) MmNEU3 sialidase over-expression in C2C12 myoblasts delays differentiation and induces hypertrophic myotube formation. J Cell Biochem 113:2967–2978
Pellegrini M, Bulzomi P, Galluzzo P, Lecis M, Leone S, Pallottini V, Marino M (2014) Naringenin modulates skeletal muscle differentiation via estrogen receptor alpha and beta signal pathway regulation. Genes Nutr 9:425
Kang JS, Choi IW, Han MH, Kim GY, Hong SH, Park C, Hwang HJ, Kim CM, Kim BW, Choi YH (2015) The cytoprotective effects of 7,8-dihydroxyflavone against oxidative stress are mediated by the upregulation of Nrf2-dependent HO-1 expression through the activation of the PI3K/Akt and ERK pathways in C2C12 myoblasts. Int J Mol Med 36:501–510
Bhukhai K, Suksen K, Bhummaphan N, Janjorn K, Thongon N, Tantikanlayaporn D, Piyachaturawat P, Suksamrarn A, Chairoungdua A (2012) A phytoestrogen diarylheptanoid mediates estrogen receptor/Akt/glycogen synthase kinase 3beta protein-dependent activation of the Wnt/beta-catenin signaling pathway. J Biol Chem 287:36168–36178
Elia D, Madhala D, Ardon E, Reshef R, Halevy O (2007) Sonic hedgehog promotes proliferation and differentiation of adult muscle cells: involvement of MAPK/ERK and PI3K/Akt pathways. Biochim Biophys Acta 1773:1438–1446
Gardner S, Anguiano M, Rotwein P (2012) Defining Akt actions in muscle differentiation. Am J Physiol Cell Physiol 303:1292–1300
Hwang J, Lee SJ, Yoo M, Go GY, Lee DY, Kim YK, Seo DW, Kang JS, Ryu JH, Bae GU (2015) Kazinol-P from Broussonetia kazinoki enhances skeletal muscle differentiation via p38MAPK and MyoD. Biochem Biophys Res Commun 456:471–475
Lluís F, Perdiguero E, Nebreda AR, Muñoz-Cánoves P (2006) Regulation of skeletal muscle gene expression by p38 MAP kinases. Trends Cell Biol 16:36–44
Lu Z, Xu S (2006) ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life 58:621–631
Ronda AC, Vasconsuelo A, Boland R (2013) 17beta-estradiol protects mitochondrial functions through extracellular-signal-regulated kinase in C2C12 muscle cells. Cell Physiol Biochem 32:1011–1023
Gonzalez I, Tripathi G, Carter EJ, Cobb LJ, Salih DA, Lovett FA, Holding C, Pell JM (2004) Akt2, a novel functional link between p38 mitogen-activated protein kinase and phosphatidylinositol 3-kinase pathways in myogenesis. Mol Cell Biol 24:3607–3622
Rommel C, Clarke BA, Zimmermann S, Nunez L, Rossman R, Reid K, Moelling K, Yancopoulos GD, Glass DJ (1999) Differentiation state-specific inhibition of the Raf–MEK–ERK pathway by Akt. Science 286:1738–1741
Trendelenburg AU, Meyer A, Rohner D, Boyle J, Hatakeyama S, Glass DJ (2009) Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am J Physiol Cell Physiol 296:1258–1270
Knight JD, Kothary R (2011) The myogenic kinome: protein kinases critical to mammalian skeletal myogenesis. Skelet Muscle 1:29
Wang Y, Hao Y, Alway SE (2011) Suppression of GSK-3beta activation by M-cadherin protects myoblasts against mitochondria-associated apoptosis during myogenic differentiation. J Cell Sci 124:3835–3847
Lovett FA, Cosgrove RA, Gonzalez I, Pell JM (2010) Essential role for p38 alpha MAPK but not p38 gamma MAPK in Igf2 expression and myoblast differentiation. Endocrinology 151:4368–4380
Trendelenburg AU, Meyer A, Jacobi C, Feige JN, Glass DJ (2012) TAK-1/p38/nNFkappaB signaling inhibits myoblast differentiation by increasing levels of Activin A. Skelet Muscle 2:3
Baeza-Raja B, Munoz-Canoves P (2004) p38 MAPK-induced nuclear factor-kappaB activity is required for skeletal muscle differentiation: role of interleukin-6. Mol Biol Cell 15:2013–2026
Bakkar N, Wang J, Ladner KJ, Wang H, Dahlman JM, Carathers M, Acharyya S, Rudnicki MA, Hollenbach AD, Guttridge DC (2008) IKK/NF-kappaB regulates skeletal myogenesis via a signaling switch to inhibit differentiation and promote mitochondrial biogenesis. J Cell Biol 180:787–802
Acknowledgements
This work is, in part, supported by Grants for Ph.D. Program to Chittipong Tipbunjong from the Office of the Higher Education Commission, Mahidol University, and National Research Universities Initiative (NRU). Also, support from The Thailand Research Fund (DBG6180030) and Center of Excellence for Innovation in Chemistry, Office of the Higher Education Commission is gratefully acknowledged. Finally, the authors would like to specially thank Prof. Karyn Esser for her technical and laboratory contributions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tipbunjong, C., Khuituan, P., Kitiyanant, Y. et al. Diarylheptanoid 1-(4-hydroxyphenyl)-7-phenyl-(6E)-6-hepten-3-one enhances C2C12 myoblast differentiation by targeting membrane estrogen receptors and activates Akt-mTOR and p38 MAPK-NF-κB signaling axes. J Nat Med 73, 735–744 (2019). https://doi.org/10.1007/s11418-019-01322-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-019-01322-7