Abstract
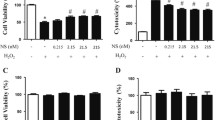
Hydroxyobtustyrene is a derivative of cinnamyl phenol isolated from Dalbergia odorifera T. Chen. The heartwood, known as ‘JiangXiang’, is a traditional Chinese medicine. Previous studies showed that hydroxyobtustyrene inhibited the biosynthesis of prostaglandins, which are mediators of neuronal cell death in ischemia. However, it currently remains unclear whether hydroxyobtustyrene protects neurons against ischemic stress. In the present study, we investigated the protective effects of hydroxyobtustyrene against sodium cyanide (NaCN)-induced chemical ischemia. Hippocampal neurons were cultured from the cerebral cortices of E18 Wistar rats. The effects of hydroxyobtustyrene on neuronal survival and trophic effects were estimated under lower and higher cell density conditions. After the treatment of 1 mM NaCN with or without hydroxyobtustyrene, an MTT assay, Hoechst staining, and immunocytochemistry for cyclooxygenase (COX)-2 were performed. Hydroxyobtustyrene increased cell viability under lower, but not normal density conditions. Neither the neurite number nor the length was influenced by hydroxyobtustyrene. NaCN significantly decreased viability and increased fragmentation in cell nuclei, and these changes were prevented by hydroxyobtustyrene. Moreover, NaCN increased the number of COX-2-positive neurons, and this was significantly prevented by the co-treatment with hydroxyobtustyrene. Therefore, hydroxyobtustyrene protected cultured hippocampal neurons against NaCN-induced chemical ischemia, which may be mediated by the inhibition of COX-2 production.





Similar content being viewed by others
References
Hotez PJ, Alvarado M, Basanez MG, Bolliger I, Bourne R, Boussinesq M, Brooker SJ, Brown AS, Buckle G, Budke CM, Carabin H, Coffeng LE, Fevre EM, Furst T, Halasa YA, Jasrasaria R, Johns NE, Keiser J, King CH, Lozano R, Murdoch ME, O’Hanlon S, Pion SD, Pullan RL, Ramaiah KD, Roberts T, Shepard DS, Smith JL, Stolk WA, Undurraga EA, Utzinger J, Wang M, Murray CJ, Naghavi M (2014) The global burden of disease study 2010: interpretation and implications for the neglected tropical diseases. PLoS Negl Trop Dis 8:e2865
Minnerup J, Sutherland BA, Buchan AM, Kleinschnitz C (2012) Neuroprotection for stroke: current status and future perspectives. Int J Mol Sci 13:11753–11772
Way JL (1984) Cyanide intoxication and its mechanism of antagonism. Annu Rev Pharmacol Toxicol 24:451–481
Carella F, Grassi MP, Savoiardo M, Contri P, Rapuzzi B, Mangoni A (1988) Dystonic-Parkinsonian syndrome after cyanide poisoning: clinical and MRI findings. J Neurol Neurosurg Psychiatry 51:1345–1348
Valenzuela R, Court J, Godoy J (1992) Delayed cyanide induced dystonia. J Neurol Neurosurg Psychiatry 55:198–199
Rosenow F, Herholz K, Lanfermann H, Weuthen G, Ebner R, Kessler J, Ghaemi M, Heiss WD (1995) Neurological sequelae of cyanide intoxication—the patterns of clinical, magnetic resonance imaging, and positron emission tomography findings. Ann Neurol 38:825–828
Rosenberg NL, Myers JA, Martin WR (1989) Cyanide-induced parkinsonism: clinical, MRI, and 6-fluorodopa PET studies. Neurology 39:142–144
Gunasekar PG, Sun PW, Kanthasamy AG, Borowitz JL, Isom GE (1996) Cyanide-induced neurotoxicity involves nitric oxide and reactive oxygen species generation after N-methyl-d-aspartate receptor activation. J Pharmacol Exp Ther 277:150–155
Bhattacharya R, Lakshmana Rao PV (2001) Pharmacological interventions of cyanide-induced cytotoxicity and DNA damage in isolated rat thymocytes and their protective efficacy in vivo. Toxicol Lett 119:59–70
Mills EM, Gunasekar PG, Li L, Borowitz JL, Isom GE (1999) Differential susceptibility of brain areas to cyanide involves different modes of cell death. Toxicol Appl Pharmacol 156:6–16
Prabhakaran K, Li L, Borowitz JL, Isom GE (2002) Cyanide induces different modes of death in cortical and mesencephalon cells. J Pharmacol Exp Ther 303:510–519
Vane JR, Bakhle YS, Botting RM (1998) Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol 38:97–120
Smith WL, DeWitt DL, Garavito RM (2000) Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem 69:145–182
Gunasekar PG, Borowitz JL, Isom GE (1998) Cyanide-induced generation of oxidative species: involvement of nitric oxide synthase and cyclooxygenase-2. J Pharmacol Exp Ther 285:236–241
Ohanian SH, Borsos T (1975) Lysis of tumor cells by antibody and complement. II. Lack of correlation between amount of C4 and C3 fixed and cell lysis. J Immunol 114:1292–1295
Kawano T, Anrather J, Zhou P, Park L, Wang G, Frys KA, Kunz A, Cho S, Orio M, Iadecola C (2006) Prostaglandin E2 EP1 receptors: downstream effectors of COX-2 neurotoxicity. Nat Med 12:225–229
Candelario-Jalil E, Fiebich BL (2008) Cyclooxygenase inhibition in ischemic brain injury. Curr Pharm Des 14:1401–1418
Ikeda-Matsuo Y, Tanji H, Narumiya S, Sasaki Y (2011) Inhibition of prostaglandin E2 EP3 receptors improves stroke injury via anti-inflammatory and anti-apoptotic mechanisms. J Neuroimmunol 238:34–43
Sun S, Zeng X, Zhang D, Guo S (2015) Diverse fungi associated with partial irregular heartwood of Dalbergia odorifera. Sci Rep 5:8464
Goda Y, Kiuchi F, Shibuya M, Sankawa U (1992) Inhibitors of prostaglandin biosynthesis from Dalbergia odorifera. Chem Pharm Bull (Tokyo) 40:2452–2457
Iwai T, Ito S, Tanimitsu K, Udagawa S, Oka J (2006) Glucagon-like peptide-1 inhibits LPS-induced IL-1β production in cultured rat astrocytes. Neurosci Res 55:352–360
Iwai T, Iinuma Y, Kodani R, Oka J (2008) Neuromedin U inhibits inflammation-mediated memory impairment and neuronal cell-death in rodents. Neurosci Res 61:113–119
Yu X, An L (2002) A serum- and antioxidant-free primary culture model of mouse cortical neurons for pharmacological screen and studies of neurotrophic and neuroprotective agents. Cell Mol Neurobiol 22:197–206
Kaech S, Banker G (2006) Culturing hippocampal neurons. Nat Protoc 1:2406–2415
Kaneko A, Sankai Y (2014) Long-term culture of rat hippocampal neurons at low density in serum-free medium: combination of the sandwich culture technique with the three-dimensional nanofibrous hydrogel PuraMatrix. PLoS One 9:e102703
Fujita R, Yoshida A, Mizuno K, Ueda H (2001) Cell density-dependent death mode switch of cultured cortical neurons under serum-free starvation stress. Cell Mol Neurobiol 21:317–324
Zhou J, Tang XC (2002) Huperzine A attenuates apoptosis and mitochondria-dependent caspase-3 in rat cortical neurons. FEBS Lett 526:21–25
Collino M, Aragno M, Mastrocola R, Gallicchio M, Rosa AC, Dianzani C, Danni O, Thiemermann C, Fantozzi R (2006) Modulation of the oxidative stress and inflammatory response by PPAR-γ agonists in the hippocampus of rats exposed to cerebral ischemia/reperfusion. Eur J Pharmacol 530:70–80
Topol EJ (2004) Failing the public health—rofecoxib, Merck, and the FDA. N Engl J Med 351:1707–1709
Solomon SD, McMurray JJ, Pfeffer MA, Wittes J, Fowler R, Finn P, Anderson WF, Zauber A, Hawk E, Bertagnolli M, Adenoma Prevention with Celecoxib Study I (2005) Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med 352:1071–1080
FitzGerald GA (2003) COX-2 and beyond: approaches to prostaglandin inhibition in human disease. Nat Rev Drug Discov 2:879–890
Hewett SJ, Bell SC, Hewett JA (2006) Contributions of cyclooxygenase-2 to neuroplasticity and neuropathology of the central nervous system. Pharmacol Ther 112:335–357
Rodrigo R, Fernández-Gajardo R, Gutiérrez R, Matamala JM, Carrasco R, Miranda-Merchak A, Feuerhake W (2013) Oxidative stress and pathophysiology of ischemic stroke: novel therapeutic opportunities. CNS Neurol Disord Drug Targets 12:698–714
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Iwai, T., Obara, K., Ito, C. et al. Hydroxyobtustyrene protects neuronal cells from chemical hypoxia-induced cell death. J Nat Med 72, 915–921 (2018). https://doi.org/10.1007/s11418-018-1224-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-018-1224-8