Abstract
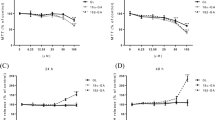
Paeoniflorin (PF), the main active component of Shaoyao-Gancao-tang, possesses significantly antinociceptive effects and many other pharmacological activities. However, its poor intestinal absorption results in low bioavailability. Therefore, enhancing PF absorption plays a vital role in exerting its therapeutic effect. Shaoyao combined with Gancao exhibited a synergistic effect. The enhancement of PF absorption through the interaction of its constituents in intestinal absorption would be greatly implicated. The present study aimed at investigating the effects of glycyrrhizin, the main constituent of Gancao, and its main metabolite, 18β-glycyrrhetinic acid (18β-GA), on the intestinal absorptive behavior of PF, and the role of P-glycoprotein (P-gp) in PF absorption using the in vitro everted rat gut sac model. The results demonstrated that 1 mM of 18β-GA significantly increased PF absorption in both the jejunum and the ileum, while 100 μM of 18β-GA only promoted the ileum absorption and had no obvious effect on the jejunum absorption. The effect of glycyrrhizin on intestinal PF absorption was related to concentrations. One mM of glycyrrhizin significantly increased PF absorption in the jejunum after 45 min and in the ileum after 90 min. But 100 μM of glycyrrhizin had an inhibitory effect in the jejunum and no effect in the ileum before 60 min. Moreover, verapamil, the well-known P-gp inhibitor, could significantly enhance the PF absorption. In conclusion, the influence of 18β-GA and glycyrrhizin on the PF absorption was related to concentrations and intestinal segments. This might be involved in the intervention of efflux transport of PF mediated by intestinal P-gp.








Similar content being viewed by others
References
Yu HY, Liu MG, Liu DN, Shang GW, Wang Y, Qi C, Zhang KP, Song ZJ, Chen J (2007) Antinociceptive effects of systemic paeoniflorin on bee venom-induced various ‘phenotypes’ of nociception and hypersensitivity. Pharmacol Biochem Behav 88(2):131–140
Jeong SJ, Lim HS, Seo CS, Kim JH, Jin SE, Yoo SR, Shin HK (2015) Traditional herbal formula Jakyakgamcho-tang (Paeonia lactiflora and Glycyrrhiza uralensis) impairs inflammatory chemokine production by inhibiting activation of STAT1 and NF-κB in HaCaT cells. Phytomedicine 22(2):326–332
Lee KK, Omiya Y, Yuzurihara M, Kase Y, Kobayashi H (2011) Antinociceptive effect of paeoniflorin via spinal α2-adrenoceptor activation in diabetic mice. Eur J Pain 15(10):1035–1039
Zhang J, Li H, Huo R, Zhai T, Li H, Sun Y, Shen B, Li N (2015) Paeoniflorin selectively inhibits LPS-provoked B-cell function. J Pharmacol Sci 128(1):8–16
Choi EM, Suh KS, Rhee SY, Kim YS (2014) Inhibitory effect of paeoniflorin on methylglyoxal-mediated oxidative stress in osteoblastic MC3T3-E1 cells. Phytomedicine 21(10):1170–1177
Dong H, Li R, Yu C, Xu T, Zhang X, Dong M (2015) Paeoniflorin inhibition of 6-hydroxydopamine-induced apoptosis in PC12 cells via suppressing reactive oxygen species-mediated PKCδ/NF-κB pathway. Neuroscience 285:70–80
Bi X, Gong M, Di L (2014) Review on prescription compatibility of Shaoyao Gancao decoction and reflection on pharmacokinetic compatibility mechanism of traditional Chinese medicine prescription based on in vivo drug interaction of main efficacious components. Evid Based Complement Alternat Med 2014:208129
Tsuji S, Yasuda K, Sumi G, Cho H, Tsuzuki T, Okada H, Kanzaki H (2012) Shakuyaku-kanzo-to inhibits smooth muscle contractions of human pregnant uterine tissue in vitro. J Obstet Gynaecol Res 38:1004–1010
Ploeger B, Mensinga T, Sips A, Meulenbelt J, DeJongh J (2000) A human physiologically-based model for glycyrrhzic acid, a compound subject to presystemic metabolism and enterohepatic cycling. Pharm Res 17(12):1516–1525
Ploeger BA, Meulenbelt J, DeJongh J (2000) Physiologically based pharmacokinetic modeling of glycyrrhizic acid, a compound subject to presystemic metabolism and enterohepatic cycling. Toxicol Appl Pharmacol 162(3):177–188
Lin SP, Tsai SY, Hou YC, Chao PD (2009) Glycyrrhizin and licorice significantly affect the pharmacokinetics of methotrexate in rats. J Agric Food Chem 57(5):1854–1859
Kao TC, Wu CH, Yen GC (2013) Glycyrrhizic acid and 18β-glycyrrhetinic acid recover glucocorticoid resistance via PI3K-induced AP1, CRE and NFAT activation. Phytomedicine 20(3–4):295–302
Jayasooriya RG, Dilshara MG, Park SR, Choi YH, Hyun JW, Chang WY, Kim GY (2014) 18β-Glycyrrhetinic acid suppresses TNF-α induced matrix metalloproteinase-9 and vascular endothelial growth factor by suppressing the Akt-dependent NF-κB pathway. Toxicol In Vitro 28(5):751–758
Kong SZ, Chen HM, Yu XT, Zhang X, Feng XX, Kang XH, Li WJ, Huang N, Luo H, Su ZR (2015) The protective effect of 18β-Glycyrrhetinic acid against UV irradiation induced photoaging in mice. Exp Gerontol 61:147–155
Chan K, Liu ZQ, Jiang ZH, Zhou H, Wong YF, Xu HX, Liu L (2006) The effects of sinomenine on intestinal absorption of paeoniflorin by the everted rat gut sac model. J Ethnopharmacol 103(3):425–432
Gong M, Wang Y, Song Y, Wang Z, Zhang Q, Wang W, Zhu J (2010) Everted intestinal sac method for quick finding absorption ingredients of Wuzhuyu decoction. Zhongguo Zhong Yao Za Zhi 35(11):1399–1404 (in Chinese)
Pan XQ, Wu YC, Gong MX, Xu YS, Wang ZM, Zhang QW, Shang YW, Lu XR, Song YF (2014) Study on pharmacological ingredients of Wuzhuyu Tang treating migraine by correlating absorption ingredients in everted intestinal sac and pharmacodynamics. Zhongguo Zhong Yao Za Zhi 39(1):126–133 (in Chinese)
Takeda S, Isono T, Wakui Y, Matsuzaki Y, Sasaki H, Amagaya S, Maruno M (1995) Absorption and excretion of paeoniflorin in rats. J Pharm Pharmacol 47(12A):1036–1040
Takara K, Ohnishi N, Horibe S, Yokoyama T (2003) Expression profiles of drug-metabolizing enzyme CYP3A and drug efflux transporter multidrug resistance 1 subfamily mRNAS in rat small intestine. Drug Metab Dispos 31(10):1235–1239
Mouly S, Paine MF (2003) P-Glycoprotein increases from proximal to distal regions of human small intestine. Pharm Res 20(10):1595–1599
Sun YM, Yang Y, Li XH, Chang MM, Li D, Pu TT, Ding X, Wang Q, Wang YL (2016) Effect of glycyrrhizic acid on the oral absorption of paeoniflorin in rats in vivo. RSC Adv 6:46925–46928
Wilson TH, Wiseman G (1954) The use of sacs of everted small intestine for the study of the transference of substances from the mucosal to the serosal surface. J Physiol 123(1):116–125
Barthe L, Bessouet M, Woodley JF, Houin G (1998) The improved everted gut sac: a simple method to study intestinal P-glycoprotein. Int J Pharm 173:255–258
Chula S, Hang L, Yinying B, Jianning S, Shi R (2012) The effects of notoginsenoside R1 on the intestinal absorption of geniposide by the everted rat gut sac model. J Ethnopharmacol 142(1):136–143
Perrone MG, Inglese C, Berardi F, Leopoldo M, Perrone R, Colabufo NA (2013) Comparative evaluation of two dye probes in the rat everted gut sac model for unambiguous classification of P-gp substrate and inhibitor. J Pharmacol Toxicol Methods 67(1):5–8
Gurunath S, Nanjwade BK, Patila PA (2014) Enhanced solubility and intestinal absorption of candesartan cilexetil solid dispersions using everted rat intestinal sacs. Saudi Pharm J 22(3):246–257
Balimane PV, Chong S, Morrison RA (2000) Current methodologies used for evaluation of intestinal permeability and absorption. J Pharmacol Toxicol Methods 44(1):301–312
Estudante M, Morais JG, Soveral G, Benet LZ (2013) Intestinal drug transporters: an overview. Adv Drug Deliv Rev 65(10):1340–1356
Schinkel AH, Jonker JW (2003) Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv Drug Deliv Rev 55(1):3–29
Varma MV, Ashokraj Y, Dey CS, Panchagnula R (2003) P-glycoprotein inhibitors and their screening: a perspective from bioavailability enhancement. Pharmacol Res 48(4):347–359
Li X, Hu J, Wang B, Sheng L, Liu Z, Yang S, Li Y (2014) Inhibitory effects of herbal constituents on P-glycoprotein in vitro and in vivo: herb–drug interactions mediated via P-gp. Toxicol Appl Pharmacol 275(2):163–175
Yoshida N, Koizumi M, Adachi I, Kawakami J (2006) Inhibition of P-glycoprotein-mediated transport by terpenoids contained in herbal medicines and natural products. Food Chem Toxicol 44(12):2033–2039
Hou YC, Lin SP, Chao PD (2012) Liquorice reduced cyclosporine bioavailability by activating P-glycoprotein and CYP 3A. Food Chem 135:2307–2312
Xu CH, Wang P, Wang Y, Yang Y, Li DH, Li HF, Sun SQ, Wu XZ (2013) Pharmacokinetic comparisons of two different combinations of Shaoyao-Gancao decoction in rats: competing mechanisms between paeoniflorin and glycyrrhetinic acid. J Ethnopharmacol 149(2):443–452
Zhao Y, Zhou G, Wang J, Jia L, Zhang P, Li R, Shan L, Liu B, Song X, Liu S, Xiao X (2013) Paeoniflorin protects against ANIT-induced cholestasis by ameliorating oxidative stress in rats. Food Chem Toxicol 58:242–248
Ma Z, Chu L, Liu H, Li J, Zhang Y, Liu W, Dai J, Yi J, Gao Y (2016) Paeoniflorin alleviates non-alcoholic steatohepatitis in rats: involvement with the ROCK/NF-κB pathway. Int Immunopharmacol 38:377–384
Chen Y, Wang J, Wang L, Chen L, Wu Q (2012) Absorption and interaction of the main constituents from the Traditional Chinese drug pair Shaoyao-Gancao via a Caco-2 cell monolayer model. Molecules 17(12):14908–14917
Liu ZQ, Jiang ZH, Liu L, Hu M (2006) Mechanisms responsible for poor oral bioavailability of paeoniflorin: role of intestinal disposition and interactions with sinomenine. Pharm Res 23(12):2768–2780
Huo X, Liu Q, Wang C, Meng Q, Sun H, Peng J, Ma X, Liu K (2013) Enhancement effect of P-gp inhibitors on the intestinal absorption and antiproliferative activity of bestatin. Eur J Pharm Sci 50(3–4):420–428
Yang C, Zhang T, Li Z, Xu L, Liu F, Ruan J, Liu K, Zhang Z (2013) P-glycoprotein is responsible for the poor intestinal absorption and low toxicity of oral aconitine: in vitro, in situ, in vivo and in silico studies. Toxicol Appl Pharmacol 273(3):561–568
Acknowledgments
This work was supported by the Capital Special Research Fund for traditional Chinese medicine in 2015 of China (1150170206/15ZY19), the Beijing project for the key members of outstanding young scientists (2015000020124G104), and partly funded by the National Natural Science Foundation of China (grant no. 81473360) and the special scientific research of traditional Chinese medicine industry in 2015 (201507002-03-01-04). We thank Siliang Fu (an English teacher at Capital Medical University) and Zhaoxia Li for the editorial assistance of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
He, R., Xu, Y., Peng, J. et al. The effects of 18β-glycyrrhetinic acid and glycyrrhizin on intestinal absorption of paeoniflorin using the everted rat gut sac model. J Nat Med 71, 198–207 (2017). https://doi.org/10.1007/s11418-016-1049-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-016-1049-2