Abstract
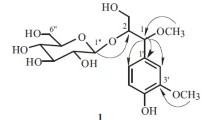
Seven new acylated sucroses, mumeoses P–V, were isolated from the flower buds of Prunus mume, cultivated in Zhejiang province, China. Their chemical structures were elucidated on the basis of chemical and physicochemical evidence. Moreover, mumeoses C, D, and R, were shown to substantially inhibit aldose reductase.


Similar content being viewed by others
References
Yoshikawa M, Murakami T, Ishikawa T, Morikawa T, Kagawa M, Higashi Y, Matsuda H (2002) New flavonol oligoglycosides and polyacylated sucroses with inhibitory effects on aldose reductase and platelet aggregation from the flowers of Prunus mume. J Nat Prod 65:1151–1155
Matsuda H, Morikawa T, Ishiwada T, Managi H, Kagawa M, Higashi Y, Yoshikawa M (2003) Medicinal flowers. VIII. Radical scavenging constituents from the flowers of Prunus mume: structure of prunose III. Chem Pharm Bull 51:440–443
Nakamura S, Fujimoto K, Matsumoto T, Nakashima S, Ohta T, Ogawa K, Matsuda H, Yoshikawa M (2013) Acylated sucroses and acylated quinic acids analogs from the flower buds of Prunus mume and their inhibitory effects on melanogenesis. Phytochemistry 92:128–136
Fujimoto K, Nakamura S, Matsumoto T, Ohta T, Ogawa K, Tamura H, Matsuda H, Yoshikawa M (2013) Medicinal flowers. XXXVIII. Structures of acylated sucroses and inhibitory effects of constituents on aldose reducatase from the flower buds of Prunus mume. Chem Pharm Bull 61:445–451
Nakamura S, Fujimoto K, Matsumoto T, Ohta T, Ogawa K, Tamura H, Matsuda H, Yoshikawa M (2013) Structures of acylated sucroses and an acylated flavonol glycoside and inhibitory effects of constituents on aldose reductase from the flower buds of Prunus mume. J Nat Med 67:799–806
Matsuda H, Hamao M, Nakamura S, Kon’i H, Murata M, Yoshikawa M (2012) Medicinal flowers. XXXIII. Anti-hyperlipidemic and anti-hyperglycemic effects of chakasaponins I-III and structure of chakasaponin IV from flower buds of Chinese Tea Plant (Camellia sinensis). Chem Pharm Bull 60:674–680
Nakamura S, Moriura T, Park S, Fujimoto K, Matsumoto T, Ohta T, Matsuda H, Yoshikawa M (2012) Melanogenesis inhibitory and fibroblast proliferation accelerating effects of noroleanane- and oleanane-type triterpene oligoglycosides from the flower buds of Camellia japonica. J Nat Prod 75:1425–1430
Nakamura S, Fujimoto K, Nakashima S, Matsumoto T, Miura T, Uno K, Matsuda H, Yoshikawa M (2012) Medicinal flowers. XXXVI. Acylated oleanane-type triterpene saponins with inhibitory effects on melanogenesis from the flower buds of Chinese Camellia japonica. Chem Pharm Bull 60:752–758
Fujimoto K, Nakamura S, Nakashima S, Matsumoto T, Uno K, Ohta T, Miura T, Matsuda H, Yoshikawa M (2012) Nor-oleanane-type and acylated oleanane-type triterpene saponins from the flower buds of Chinese Camellia japonica and their inhibitory effects on melanogenesis. Chem Pharm Bull 60:1188–1194
Nakamura S, Nakashima S, Tanabe G, Oda Y, Yokota N, Fujimoto K, Matsumoto T, Sakuma R, Ohta T, Ogawa K, Nishida S, Miki H, Matsuda H, Muraoka O, Yoshikawa M (2013) Alkaloid constituents from flower buds and leaves of sacred lotus (Nelumbo nucifera, Nymphaeaceae) with melanogenesis inhibitory activity in B16 melanoma cells. Bioorg Med Chem 21:779–787
Liu J, Nakamura S, Matsuda H, Yoshikawa M (2013) Hydrangeamines A and B, novel polyketide-type pseudoalkaloid-coupled secoiridoid glycosides from the flowers of Hydrangea macrophylla var. thunbergii. Tetrahedron Lett 54:32–34
Liu J, Nakamura S, Zhuang Y, Yoshikawa M, Hussein GME, Matsuo K, Matsuda H (2013) Medicinal flowers. XXXX. Structures of dihydroisocoumarin glycosides and inhibitory effects on aldose reductase from the flowers of Hydrangea macrophylla var. thunbergii. Chem Pharm Bull 61:655–661
Tanaka T, Nakashima T, Ueda T, Tomii K, Kouno I (2007) Facile discrimination of aldose enantiomers by reversed-phase HPLC. Chem Pharm Bull 55:899–901
Yoshinari K, Sashida Y, Mimaki Y, Shimamura M (1990) New polyacylated sucrose derivatives from the bark of Prunus padus. Chem Pharm Bull 38:415–417
Matsuda H, Morikawa T, Toguchida I, Harima S, Yoshikawa M (2002) Medicinal flowers. VI. Absolute stereostructures of two new flavanone glycosides and a phenylbutanoid glycoside from the flowers of Chrysanthemum indicum L.: their inhibitory activities for rat lens aldose reductase. Chem Pharm Bull 50:972–975
Matsuda H, Morikawa T, Toguchida I, Yoshikawa M (2002) Structural requirements of flavonoids and related compounds for aldose reductase inhibitory activity. Chem Pharm Bull 50:788–795
Morikawa T, Kishi A, Pongpiriyadacha Y, Matsuda H, Yoshikawa M (2003) Structures of new friedelane-type triterpenes and eudesmane-type sesquiterpene and aldose reductase inhibitors from Salacia chinensis. J Nat Prod 66:1191–1196
Xie H, Wang T, Matsuda H, Morikawa T, Yoshikawa M, Tani T (2005) Bioactive constituents from Chinese natural medicines. XV. Inhibitory effect on aldose reductase and structures of saussureosides A and B from Saussurea medusa. Chem Pharm Bull 53:1416–1422
Morikawa T, Xie H, Wang T, Matsuda H, Yoshikawa M (2008) Bioactive constituents from Chinese natural medicines. XXXII. Aminopeptidase N and aldose reductase inhibitors from Sinocrassula indica: structures of sinocrassosides B4, B5, C1, and D1–D3. Chem Pharm Bull 56:1438–1444
Matsuda H, Asao Y, Nakamura S, Hamao M, Sugimoto S, Hongo M, Pongpiriyadacha Y, Yoshikawa M (2009) Antidiabetogenic constituents from the Thai traditional medicine Cotylelobium melanoxylon. Chem Pharm Bull 57:487–494
Acknowledgments
This research was supported in part by a Ministry of Education, Culture, Sports, Science and Technology (MEXT)-Supported Program for the Strategic Research Foundation at Private Universities, and by a Grant-in-Aid for Young Scientists (B) from the Japan Society for the Promotion of Science (JSPS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fujimoto, K., Nakamura, S., Matsumoto, T. et al. Structures of acylated sucroses from the flower buds of Prunus mume . J Nat Med 68, 481–487 (2014). https://doi.org/10.1007/s11418-014-0818-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-014-0818-z