Abstract
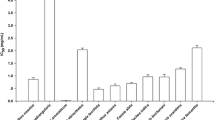
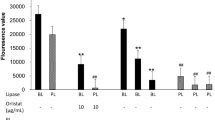
The present study investigated inhibition of pancreatic lipase and metabolic effects of high caloric diet in rats. The Passiflora nitida hydroethanol leaf extract (PNE) was used in in vitro assays or administered to rats to study dyslipidemia. Inhibition of lipase in vitro was studied by a spectrophotometric assay using orlistat as the positive control. The effects of PNE on reduction of postprandial triglyceride were studied by oral fat-overloading in rats. Metabolic alterations were induced using the cafeteria diet and 4 weeks post-treatment with PNE or orlistat and blood samples were collected and biochemical analyses were performed. Liver and retroperitoneal fat tissues were obtained to analyze weight and steatosis. IC50 (μg/mL) values for pancreatic lipase inhibition were 21.2 ± 0.8 and 0.1 ± 0.01 for PNE and orlistat, respectively. Oral administration of lipid emulsion resulted in postprandial hypertriglyceridemia at 3 h postadministration and when rats were then administered PNE and orlistat there was decreased of triglyceride levels by 15 % compared to control. Although the energy consumption by the cafeteria diet had been higher, there was no significant weight gain observed in the study groups. The cafeteria diet resulted in a significant increase of weight in the retroperitoneal fat and hypertriglyceridemia levels that could be significantly reduced by PNE and orlistat treatment. We hypothesized that PNE administration prevented the hypertriglyceridemia in rats with a high caloric diet, possibly owing to reduction of lipid absorption and pancreatic lipase inhibition.




Similar content being viewed by others
References
Adisakwattana S, Moonrat J, Srichairat S, Chanasit C, Tiraponporn H, Chanathong B, Ngamukote S, Makynen K, Sapwarobol S (2010) Lipid-lowering mechanisms of grape seed extract (Vitis vinifera L) and its antihyperlidemic activity. J Med Plants Res 4:2113–2120
França E, Alves JGB (2006) Dislipidemia entre crianças e adolescentes de Pernambuco. Arq Bras Cardiol 87:722–727
Robins SJ, Lyass A, Zachariah JP, Massaro JM, Vasan RS (2011) Insulin resistance and the relationship of a dyslipidemia to coronary heart disease: the Framingham Heart Study. Arterioscler Thromb Vasc Biol 31:1208–1214
Gupta H, Pawar D, Riva A, Bombardelli E, Morazzoni P (2012) A randomized, double-blind, placebo-controlled trial to evaluate efficacy and tolerability of an optimized botanical combination in the management of patients with primary hypercholesterolemia and mixed dyslipidemia. Phytother Res 26:265–272
Garza AL, Milagro FI, Boque N, Campión J, Martínez JA (2011) Natural inhibitors of pancreatic lipase as new players in obesity treatment. Planta Med 77(8):773–785
Tucci SA, Boyland EJ, Halford JCG (2010) The role of lipid and carbohydrate digestive enzyme inhibitors in the management of obesity: a review of current and emerging therapeutics agents. Diabetes Metab Syndr Obes 3:125–143
Prasad H, Ryan DA, Celzo MF, Stapleton D (2012) Metabolic syndrome: definition and therapeutic implications. Postgrad Med 124:21–30
Psaty BM, Rivara FP (2012) Universal screening and drug treatment of dyslipidemia in children and adolescents. JAMA 307:257–258
Oliveira VB, Yamada LT, Fagg CW, Brandão MGL (2012) Native foods from Brazilian biodiversity as a source of bioactive compounds. Food Res Int 48:170–179
Dembitsky VM, Poovarodom S, Leontowicz H, Leontowicz M, Vearasilp S, Trakhtenberg S, Gorinstein S (2011) The multiple nutrition properties of some exotic fruits: biological activity and active metabolites. Food Res Int 44:1671–1701
Beraldo J, Kato ETM (2010) Morfoanatomia de folhas e caules de Passiflora edulis Sims, Passifloraceae. Rev Bras Farmacog 20:233–239
Zucolotto SM, Palermo JAE, Schenkel EP (2006) Estudo fitoquímico das raízes de Passiflora edulis forma flavicarpa Degener. Acta Farm Bonaerense 25:5–9
Abreu PP, Souza MM, Santos EA, Pires MV, Pires MM, Almeida AAF (2009) Passion flower hybrids and their use in the ornamental plant market: perspectives for sustainable development with emphasis on Brazil. Euphytica 166:307–315
Carvalho MJ, Pedrosa TN, Guilhon-Simplicio F, Nunez CV, Ohana DT, Pereira MM, Lima ES (2010) Estudo farmacognóstico e atividade in vitro sobre a coagulação sanguínea e agregação plaquetária das folhas de Passiflora nitida Kunth (Passifloraceae). Acta Amaz 40:199–206
McDougall GJ, Kulkarni NN, Stewart D (2009) Berry polyphenols inhibit pancreatic lipase activity in vitro. Food Chem 115:193–199
Pastore AP, Cesaretti MLR, Ginoza M, Voltera AL, Kohlmann JO (2010) Effects of the association of experimental neuroendocrine and exocrine obesity on tail blood pressure and glucose metabolism in Wistar rats. J Bras Nefrol 32:195–200
Helrich K (1990) Methods 920.39 and 978.04. In: AOAC: Official methods of analysis, vol 1. Association of Official Analytical Chemists, Inc., Arlington
Núcleo de Estudos e Pesquisas em Alimentação (2006). Tabela brasileira de composição de alimentos: versão II. Campinas: NEPA-UNICAMP 105p
FAO Food and Agriculture Organization of the United Nations and LATINFOODS (2009). Tabla de Composición de Alimentos de América Latina. http://www.rlc.fao.org/es/conozca-fao/que-hace-fao/estadisticas/composicion-alimentos/componentes. Accessed Dec 2012
Jang HH, Park MY, Kim HW, Lee YM, Hwang KA, Park JH, Park DS, Kwon O (2012) Black rice (Oryza sativa L.) extract attenuates hepatic steatosis in C57BL/6J mice fed a high-fat diet via fatty acid oxidation. Nutr Metab 9:27–38
Aizza LCB, Dornelas MC (2011) A genomic approach to study anthocyanin synthesis and flower pigmentation in passion flowers. J Nucleic Acids 2011:1–17
Bendini A, Cerretani L, Pizzolante L, Toschi TG, Guzzo F, Ceoldo S, Marconi AM, Levi FAM (2006) Phenol content related to antioxidant and antimicrobial activities of Passiflora spp. extracts. Eur Food Res Technol 223:102–109
Galleano M, Calabro V, Prince PD, Litterio MC, Potrkowski B, Vazquez-Prieto MA, Miatelo RM, Oteiza PI, Fraga CG (2012) Flavonoids and metabolic syndrome. Ann NY Acad Sci 1259:87–94
Pizziolo VR, Brasileiro BG, Oliveira TT, Vagem TJ (2011) Plantas com possível atividade hipolipidêmica: uma revisão bibliográfica de livros editados no Brasil entre 1998 e 2008. Rev Bras Plantas Med 13(1):98–109
Sashidhara KV, Kumara A, Kumara M, Sonkarb R, Bhatia G, Khanna AK (2010) Novel coumarin derivatives as potential antidyslipidemic agents. Bioorg Med Chem Lett 20:4248–4251
Eri EM, Murray WH (2012) Citrus flavonoids and the prevention of atherosclerosis. Cardiovasc Haematol Disord 12:84–91
Roh C, Jung U (2012) Screening of crude plant extracts with anti-obesity activity. Int J Mol Sci 13:1710–1719
Souza SP, Pereira LLS, Souza AA, Santos CD (2011) Inhibition of pancreatic lipase by extracts of Baccharis trimera: evaluation of antinutrients and effect on glycosidases. Braz J Pharmacogn 21:450–455
Zheng CD, Duan YQ, Gao JM, Ruan ZG (2010) Screening for anti-lipase properties of 37 traditional Chinese medicinal herbs. J Chin Med Assoc 73:319–324
Conforti F, Perri V, Menichni F, Marrelli M, Uzunov D, Statti GA, Menichini F (2012) Wild Mediterranean dietary plants as inhibitors of pancreatic lipase. Phytother Res 26:600–604
Kaewpiboon C, Lirdprapamongkol K, Srisomsap C, Winayanuwattikun P, Yongvanich T, Puwaprisirisan P, Svasti J, Assavalapsakul W (2012) Studies of the in vitro cytotoxic, antioxidant, lipase inhibitory and antimicrobial activities of selected Thai medicinal plants. BMC Complement Altern Med 12:217–224
Ramírez G, Zavala M, Pérez J, Zamilpa A (2012) In vitro screening of medicinal plants used in Mexico as antidiabetics with glucosidase and lipase inhibitory activities. J Evid Based Complementary Altern Med 2012:1–6
Kiage-Mokua BN, Roos N, Schrezenmeir J (2012) Lapacho tea (Tabebuia impetiginosa) extract inhibits pancreatic lipase and delays postprandial triglyceride increase in rats. Phytother Res 26:1878–1883
Ramírez-Vélez R (2011) La lipemia pos-prandial induce disfunción endotelial y mayor grado de resistencia a la insulina en sujetos sanos. Endocr Nutr 58:74–79
Chidrawar VR, Patel KN, Bothta SB, Shiromwar SS, Koli AR, Kalyankar GG (2012) Anti-obesity effect of Stellaria media methanolic extract in the murine model of cafeteria diet induced obesity. Int J Nutr Pharmacol Neurol Dis 2:121–131
Pinto-Junior DAC, Seraphim PM (2012) Cafeteria diet intake for fourteen weeks can cause obesity and insulin resistance in Wistar rats. Rev Nutr 25:313–319
Sampey BP, Vanhoose AM, Winfield HM, Freemerman AJ, Muehlbauer MJ, Fueger PT, Newgard CB, Makowski L (2011) Cafeteria diet is a robust model of human metabolic syndrome with liver and adipose inflammation: comparison to high-fat diet. Obesity 19:1109–1117
Kumar S, Alagawadi KR, Rao MR (2011) Effect of Argyreia speciosa root extract on cafeteria diet-induced obesity in rats. Indian J Pharmacol 43:163–167
Al-Awwadi NAJ, Alyassin FF (2011) Resveratrol reduce metabolic disorders in obese rats. J Pharm Biomed Sci 1:167–173
Patil A, Thakurdesai PA, Pawar S, Soni K (2012) Evaluation of ethanolic leaf extract of Ceiba pentandra for anti-obesity and hypolipidaemic activity in cafeteria diet (CD) treated Wistar albino rats. Int J Pharma Sci Res 3:2664–2668
Waterlow JC (2006) Protein turnover. CABI, Wallingford
Panchal SK, Poudyal H, Iyer A, Nazer R, Alan A, Diwan V, Kauter K, Conrad S, Campbell F, Ward L, Gode G, Fenning A, Brown L (2012) High-carbohydrate high-fat-diet-induced metabolic syndrome and cardiovascular remodeling in rats. J Cardiovasc Pharmacol 57:51–64
Mathieu P, Poirier P, Pibarot P, Lemieuse I, Després JP (2009) Visceral obesity: the link among inflammation, hypertension, and cardiovascular disease. Hypertension 53:577–584
Madeira FB, Silva AA, Veloso HF, Goldani MZ, Kac G, Cardoso VC, Bettiol H, Barbier MA (2013) Normal weight obesity is associated with metabolic syndrome and insulin resistance in young adults from a middle-income country. PLoS One 8:1–9
Speaker KJ, Fleshner M (2012) Interleukin-1 beta: a potential link between stress and the development of visceral obesity. BMC Physiol 12:8
Amacher DE, Schomaker S, Burkhardt JE (1998) The relationship among microsomal enzyme induction, liver weight and histological change in rat toxicology studies. Food Chem Toxicol 36:9–10
Roe JCF (ed) (1970) Metabolic aspects of food safety. Academic, New York
Robert MR, Katsuhiko Y, Abraham N, Takanori H, Gordon F (2010) Hepatic enzyme induction: histopathology. Toxicol Pathol 38:776–795
Cohen JC, Horton JD, Hobbs HH (2011) Human fatty liver disease: old questions and new insights. Science 332:1519–1523
Park HJ, DiNatale DA, Chung MY, Park YK, Lee JY, Koo SI, O’Connor M, Manautou JE, Bruno RS (2011) Green tea extract attenuates hepatic steatosis by decreasing adipose lipogenesis and enhancing hepatic antioxidant defenses in ob/ob mice. J Nutr Biochem 22:393–400
Park HJ, Jung UJ, Lee MK, Cho SJ, Jung HK, Hong JH, Park YB, Kim SR, Shim S, Jung J, Choi MS (2012) Modulation of lipid metabolism by polyphenol-rich grape skin extract improves liver steatosis and adiposity in high fat fed mice. Mol Nutr Food Res 00:1–5
Pak W, Takayama F, Hasegawa A, Mankura M, Egashira T, Ueki K, Nakamoto K, Kawasaki H, Mori A (2012) Water extract of Vitis coignetiae Pulliat leaves attenuates oxidative stress and inflammation in progressive NASH rats. Acta Med Okayama 66:317–327
Takayama F, Nakamoto K, Kawasaki H, Mankura M, Egashira T, Ueki K, Hasegawa A, Okada S, Mori A (2009) Beneficial effects of Vitis coignetiae Pulliat leaves on nonalcoholic steatohepatitis in a rat model. Acta Med Okayama 63:105–111
Acknowledgments
The authors are grateful to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado do Amazonas (FAPEAM) for financial support of this research. ESL is a member of the INCT de Processos Redox em Biomedicina-Redoxoma (MCT/CNPq). APAB received a grant from DCR/CNPq/FAPEAM.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Teixeira, L.S., Lima, A.S., Boleti, A.P.A. et al. Effects of Passiflora nitida Kunth leaf extract on digestive enzymes and high caloric diet in rats. J Nat Med 68, 316–325 (2014). https://doi.org/10.1007/s11418-013-0800-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-013-0800-1