Abstract
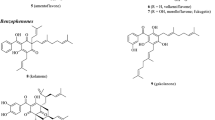
Four new compounds, impecylone (1), deacetylimpecyloside (2), seguinoside K 4-methylether (3) and impecylenolide (4), were isolated from Imperata cylindrica along with two known compounds, impecyloside (5) and seguinoside K (6). Their structures were elucidated mainly by spectroscopic analyses including 1D- and 2D-NMR techniques, and the absolute configuration of 1 was confirmed by X-ray diffraction analysis. In calcium assay, the result indicated that compounds 1, 2, 4 and 5 cannot obviously inhibit the calcium peak value compared with the negative control, and suggested that the four compounds could not have anti-inflammatory activity.





Similar content being viewed by others
References
Editorial Committee of Flora of China, Chinese Academy of Sciences (1997) Flora of China. Science Press, Beijing, no. 10, p 31
Chinese Pharmacopoeia Commission (2010) Chinese Pharmacopoeia. People’s Medical Publishing House, Beijing, no I, p 99
Wang ML, Wang SX, Sun QS (1997) Advancement of chemical and pharmacological studies on Imperata cylindrica Beauv. Var. major (Nees) C. E. Hubb. J Shenyang Pharm Univ 14:67–69
Matsunaga K, Shibuya M, Ohizumi Y (1994) Graminone B, a novel lignan with vasodilative activity from Imperata cylindrica. J Nat Prod 57:1734–1736
Yoon JS, Lee MK, Sung SH, Kim YC (2006) Neuroprotective 2-(2-phenylethyl)chromones of Imperata cylindrica. J Nat Prod 69:290–291
Lee DY, Han KM, Song MC, Lee DG, Rho YD, Baek NI (2008) A new lignan glycoside from the rhizomes of Imperata cylindrica. J Asian Nat Prod Res 10:299–302
Zhong XN, Otsuka H, Ide T, Hirata E, Takeda Y (1999) Hydroquinone diglycoside acyl esters from the leaves of Myrsine seguinii. Phytochemistry 52:923–927
Jiang DS, Chiaro C, Maddali P, Prabhu KS, Peterson DG (2009) Identification of hydroxycinnamic acid–Maillard reaction products in low-moisture baking model systems. J Agric Food Chem 57:9932–9943
Sun K, Li X, Li W, Wang JH, Liu JM, Sha Y (2004) Two new lactones and one new aryl-8-oxa-bicyclo(3,2,1)oct-3-en-2-one from Descurainia sophia. Chem Pharm Bull 52:1483–1486
Ceorgopoulou C, Aligiannis N, Fokialakis N, Mitaku S (2005) Acretoside, a new sucrose ester from Aristolochia cretica. J Asian Nat Prod Res 7:799–803
Kim E, Lee HK, Hwang EI, Kim SU, Lee WS, Lee SK (2005) Stereochemistry of phellinsin A: a concise synthesis of α-arylidene-γ-lactones. Synth Commun 35:1231–1238
Mali RS, Babu KN (2002) Efficient synthesis of α-benzylidene-γ-methyl-γ-butyrolactones. Helv Chim Acta 85:3525–3531
Acknowledgments
The project was financially supported by the Shanghai Science and Technology Development Foundation (10DZ1970200) and the Program for Changjiang Scholars and Innovative Research Team in University (IRT1071).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, X., Zhang, BF., Yang, L. et al. Four new compounds from Imperata cylindrica . J Nat Med 68, 295–301 (2014). https://doi.org/10.1007/s11418-013-0793-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-013-0793-9