Abstract
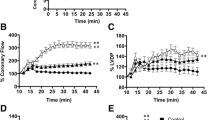
This investigation was aimed to probe the pharmacological base of medicinal use of Acorus calamus in ischemic heart diseases. Effect on heart parameters was studied in isolated rabbit heart while coronary vasodilator effect was studied in isolated bovine coronary arterial rings, suspended in tissue baths filled with Krebs solution, maintained at 37°C, aerated with carbogen and responses were measured on PowerLab data acquisition system. In Langendorrf’s perfused rabbit heart, the crude extract of Acorus calamus (Ac.Cr) at 0.01–10 mg/mL partially suppressed force of ventricular contractions (FVC), heart rate (HR) and coronary flow (CF). The ethylacetate fraction completely suppressed FVC, partially suppressed HR and CF, while the nHexane fraction exhibited similar effect on FVC and HR but increased CF, similar to methacholine and arachidonic acid. In bovine coronary arterial preparations, Ac.Cr caused inhibition of U46619 (20 nM)-precontractions, which was blocked in presence of increasing concentration of K+ (4.8–20 mM), tetraethylammonium (1 μM) and SKF525A (10 μM), similar to arachidonic acid and methacholine, indicating K+ channels activation and possible involvement of endothelial-derived hyperpolarizing factor (EDHF). Activity-directed fractionation revealed that EDHF-mediated activity is concentrated in the nHexane fraction. When tested against high K+, the ethylacetate fraction was found more potent than parent crude extract and nHexane fraction. These data indicate that Ac.Cr mediates coronary vasodilator effect primarily through EDHF, responsible for the increase in CF, while the cardiac depressant effects may be due to the presence of additional cardiac depressant constituent(s), thus provides possible mechanistic basis to its medicinal use in ischemic heart diseases.





Similar content being viewed by others
References
Wiart C (2002) Medicinal plants of South East Asia. Prentice Hall, Selangor
Wallis TE (1985) Text book of pharmacognosy. CBS Publishers & Distributors, New Delhi
Duke JA, Bogenschutz-Godwin MJ, Du celliar J, Duke PK (2002) Hand book of medicinal herbs, 2nd edn. CRC Press, Boca Raton
Baquar SR (1989) Medicinal and poisonous plants of Pakistan. Printas, Karachi
Kapoor LD (2000) Handbook of Ayurvedic medicinal plants. CRC Press, Boca Raton
Danilevskii NF, Antonishin BV (1982) Antimicrobial activity of a tincture of Japanese pagoda tree (Sophora japonica) and of the essential oil of sweet flag (Acorus calamus). Mikrobiol Zh 44:80–82
Shoba FG, Thomas M (2001) Study of antidiarrhoeal activity of four medicinal plants in castor-oil induced diarrhoea. J Ethnopharmacol 76:73–76
Parab RS, Mengi SA (2002) Hypolipidemic activity of Acorus calamus L. in rats. Fitoterapia 73:451–455
Shukla PK, Khanna VK, Ali MM, Maurya RR, Handa SS, Srimal RC (2002) Protective effect of Acorus calamus against acrylamide induced neurotoxicity. Phytother Res 16:256–260
Acuna UM, Atha DE, Ma J, Nee MH, Kennelly EJ (2002) Antioxidant capacities of ten edible North American plants. Phytother Res 16:63–65
Oh MH, Houghton PJ, Whang WK, Cho JH (2004) Screening of Korean herbal medicines used to improve cognitive function for anti-cholinesterase activity. Phytomedicine 11:544–548
Jain N, Jain R, Jain A, Jain DK, Chandel HS (2010) Evaluation of wound-healing activity of Acorus calamus Linn. Nat Prod Res 24:534–541
Gilani AH, Shah AJ, Manzoor A, Farzana S (2006) Antispasmodic effect of Acorus calamus Linn. is mediated through calcium channel blockade. Phytother Res 20:1080–1084
Shah AJ, Gilani AH (2009) Blood pressure lowering and vascular modulator effects of Acorus calamus extract are mediated through multiple pathways. J Cardiovasc Pharmacol 54:38–46
Williamson EM, Okpako DT, Evans FJ (1998) Pharmacological methods in phytotherapy research. Wiley, Chichester
Venkatesh S, Madhava RB, Dayanand RG, Mullangi R, Lakshman M (2010) Antihyperglycemic and hypolipidemic effects of Helicteres isora roots in alloxan-induced diabetic rats: a possible mechanism of action. J Nat Med 64:295–304
Wang Y, Han T, Zhu Y, Zheng CJ, Ming QL, Rahman K, Qin LP (2010) Antidepressant properties of bioactive fractions from the extract of Crocus sativus L. J Nat Med 64:24–30
National Research Council (1996) Guide for the care and use of laboratory animals. National Academy Press, Washington
Balderston SM, Johnson KE, Reiter MJ (1991) Electrophysiologic evaluation of cardiovascular agents in the isolated intact rabbit heart. J Pharmacol Methods 25:205–213
Taqvi SIH, Ghayur MN, Gialni AH, Saify ZS, Aftab MT (2006) Synthesis and smooth muscle-selective relaxant activity of a piperidine analogue: 1-(4′-fluorophenacyl)-4-hydroxy-4-phenylpiperidinium chloride. Arch Pharm Res 29:34–39
Stephen F, Malcolm JL (1993) A factor released from coronary vascular endothelium inhibits myocardial contractile performance. Am J Physiol Heart Circ Physiol 264:H830–H836
Campbell WB, Gebremedhin D, Pratt PF, Harder DR (1996) Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res 78:415–423
Farre AJ, Columbo M, Fort M, Gutierrez B (1991) Differential effects of various Ca2+ antagonists. Gen Pharmacol 22:177–181
Fantel AG, Nekahi N, Shepard TH, Cornel LM, Unis AS, Lemire RG (1997) The teratogenicity of N G-nitro-l-arginine methyl ester (l-NAME), a nitric oxide synthase inhibitor in rats. Reprod Toxicol 11:709–717
Moncada S, Korbut R, Bunting S, Vane JR (1978) Prostacyclin is a circulating hormone. Nature 273:767–768
Fleckenstein A (1977) Specific pharmacology of Ca2+ in myocardium, cardiac pacemakers and vascular smooth muscle. Rev Pharmacol Toxicol 17:149–166
Bolton TB (1979) Mechanism of action of transmitters and other substances on smooth muscles. Physiol Rev 59:606–718
Rang HP, Dale MM, Ritter JM (1995) Pharmacology. Churchill Livingstone, London
Feigl EO (1983) Coronary physiology. Physiol Rev 63:1–205
Bova S, Cargnelli G, D’Amato E, Forti S, Yang Q, Trevisi L, Debetto P, Cima L, Luciani S, Padrini R (1997) Calcium-antagonist effects of norbormide on isolated perfused heart and cardiac myocytes of guinea-pig: a comparison with verapamil. Br J Pharmacol 120:19–24
Feletou M, Vanhoutte PM (1988) Endothelium-dependent hyper-polarization of canine coronary smooth muscle. Br J Pharmacol 93:515–524
Qian YZ, Levasseur JE, Toshida KI, Kukreja RC (1996) KATP channels in rat heart: blockade of ischemic and acetylcholine-mediated preconditioning by glibenclamide. Am J Physiol 271:H23–H28
Huang JS, Ramamurthy SK, Lin X, Le BGC (2004) Cell signaling through thromboxane A2 receptors. Cell Signal 16:521–533
Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH (1998) K1 is an endothelium-derived hyperpolarizing factor in rat arteries. Nature 396:269–272
Ross GR, Yallampalli C (2006) Endothelium-independent relaxation by adrenomedullin in pregnant rat mesenteric artery: role of cAMP-dependent protein kinase A and calcium-activated potassium channels. JPET 317:1269–1275
Dora KA, Garland CJ (2001) Properties of smooth muscle hyperpolarization and relaxation to potassium in the rat isolated mesenteric artery. Am J Physiol Heart Circ Physiol 280:H2424–H2429
Silvia N, Wiliam SW, Hilary L, Andrea L, Susan M, Andrew GP, Wiliam M (2003) Evaluation of potassium ion as the endothelium-derived hyperpolarizing factor (EDHF) in the bovine coronary artery. Br J Pharmacol 139:982–988
Rosolowsky M, Campbell WB (1993) Role of PGI2 and EETs in the relaxation of bovine coronary arteries to arachidonic acid. Am J Physiol 264:H327–H335
Gebremedhin D, Harder DR, Pratt PF, Campbell WB (1998) Bioassay of an endothelium-derived hyperpolarizing factor from bovine coronary arteries: role of a cytochrome P450 metabolite. J Vasc Res 35:274–284
Fujii K, Tominaga M, Ohmori S, Kobayashi K, Koga T, Takata Y, Fumjishima M (1992) Decreased endothelium-dependent hyperpolarization to acetylcholine in smooth muscle of the mesenteric artery of spontaneously hypertensive rats. Circ Res 70:660–669
Trautwein W (1973) Membrane currents in cardiac muscle fibres. Physiol Rev 53:793–835
Holzmann S, Kukovetz WR, Windischhofer W, Paschke E, Graier WF (1994) Pharmacologic differentiation between endothelium-dependent relaxations sensitive and resistant to nitro-l-arginine in coronary arteries. J Cardiovasc Pharmacol 23:747–756
Cohen RA, Vanhoutte PM (1995) Endothelium-dependent hyperpolarization-beyond nitric oxide and cyclic GMP. Circulation 92:3337–3349
Acknowledgments
This study was carried out with partial support from the Pakistan Science Foundation. The authors are thankful to Mr. Stephen Hudson, Department of Biochemistry, Medical College of Wisconsin, USA, for the grammatical and typographical corrections in the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shah, A.J., Gilani, A.H. Aqueous-methanolic extract of sweet flag (Acorus calamus) possesses cardiac depressant and endothelial-derived hyperpolarizing factor-mediated coronary vasodilator effects. J Nat Med 66, 119–126 (2012). https://doi.org/10.1007/s11418-011-0561-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-011-0561-7