Abstract
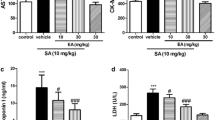
The effect of ethanol extract of Phyllanthus maderaspatensis (PME), a popular south Indian dietary supplement, was studied for its chemoprotective property on adriamycin (ADR)-induced toxicity and oxidative stress in mice. Adriamycin toxicity was evaluated biochemically by measuring the serum concentration of lactate dehydrogenase (LDH) and creatinine phosphokinase (CPK). Genotoxicity was evaluated by measuring the frequency of micronucleated polychromatic erythrocytes (MNPCEs) in bone marrow cells. Oxidative stress in the heart tissue was estimated by measuring the glutathione (GSH) levels in the homogenate. The treatment of mice with different doses of PME (400 mg/kg and 600 mg/kg body weight, p.o.,) for 7 days before the administration of a single i.p. dose of ADR (15 mg/kg) exhibited significant protection in a dose-dependent manner. The results clearly indicate that PME has a protective effect against ADR-induced toxicity, as revealed by the decrease in the concentrations of LDH, CPK, and the frequency of MNPCEs. The increased levels of GSH are indicative of the antioxidant property of PME.




Similar content being viewed by others
References
Weijl NI, Cleton FJ, Osanto S (1997) Free radicals and antioxidants in chemotherapy-induced toxicity. Cancer Treat Rev 23:209–240
Thyagarajan SP, Subramanian S, Thirunalasundari T, Venkateswaran PS, Blumberg BS (1988) Effect of Phyllanthus amarus on chronic carriers of hepatitis B virus. Lancet 2:764–766
Unander DW, Venkateswaran PS, Millman I, Bryan HH, Blumberg BS (1990) Phyllanthus species: source of new antiviral compounds. In: Janick J, Simon JE (eds) Advances in new crops. Timber Press, Portland, Oregon, pp 518–521
Unander DW, Webster GL, Blumberg BS (1995) Usage and bioassays in Phyllanthus (Euphorbiaceae). IV. Clustering of antiviral uses and other effects. J Ethnopharmacol 45:1–18
Kirthikar KR, Basu BD (1999) In: Blatter E, Calus JF, Mhaskar KS (eds) Indian medicinal plants. International book distributors. Dehradun, India, pp 2222–2223
Asha VV, Pushpangadan P (1998) Preliminary evaluation of the antihepatotoxic activity of Phyllanthus kozhikodianus, P. maderaspatensis and Solanum indicum. Fitoterapia 69:255–259
Asha VV, Akhila S, Wills PJ, Subramoniam A (2004) Further studies on the antihepatotoxic activity of Phyllanthus maderaspatensis Linn. J Ethnopharmacol 92:67–70
International Agency for Research on Cancer (IARC) (1987) IARC monographs on the evaluation of carcinogenic risks to human, vol 42. IARC, Lyon, France
Sorsa M, Hemminki K, Vainio H (1985) Occupational exposure to anticancer drugs—potential and real hazards. Mutat Res 154:135–149
Gewirtz DA (1999) A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol 57:727–741
Gruber BM, Anuszewska EL, Skierski JS (2001) Activation of programmed cell death (apoptosis) by adriamycin in human neoplastic cells. Mutat Res 484:87–93
Myers CE, McGuire WP, Liss RH, Ifrim I, Grotzinger K, Young RC (1977) Adriamycin: the role of lipid peroxidation in cardiac toxicity and tumor response. Science 197:165–167
Marquardt H, Philips FS, Sternberg SS (1976) Tumorigenicity in vivo and induction of malignant transformation and mutagenesis in cell cultures by adriamycin and daunomycin. Cancer Res 36:2065–2069
Rosselli F, Zaccaro L, Venturi M, Rossi AM (1990) Persistence of drug-induced chromosome aberrations in peripheral blood lymphocytes of the rat. Mutat Res 232:107–114
Cook NC, Samman S (1996) Flavonoids—chemistry, metabolism, cardioprotective effects, and dietary sources. J Nutr Biochem 7:66–76
Jagetia GC, Jacob PS (1992) Vinblastine treatment induces dose-dependent increases in the frequency of micronuclei in mouse bone marrow. Mutat Res 280:87–92
Schmid W (1975) The micronucleus test. Mutat Res 31:9–15
Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25:192–205
Myers CE, Chabner BA (1990) Anthracyclines. In: Chabner BA, Collins JM (eds) Cancer chemotherapy: principles and practice. Lippincott, Philadelphia, Pennsylvania, pp 356–381
Rajagopalan S, Politi PM, Sinha BK, Myers CE (1988) Adriamycin-induced free radical formation in the perfused rat heart: implications for cardiotoxicity. Cancer Res 48:4766–4769
Lee V, Randhawa AK, Singal PK (1991) Adriamycin-induced myocardial dysfunction in vitro is mediated by free radicals. Am J Physiol Heart Circ Physiol 261:H989–H995
Fantone JC, Ward PA (1982) Role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am J Pathol 107:395–418
Fox RB (1984) Prevention of granulocyte-mediated oxidant lung injury in rats by a hydroxyl radical scavenger, dimethylthiourea. J Clin Invest 74:1456–1464
Varani J, Fligiel SE, Till GO, Kunkel RG, Ryan US, Ward PA (1985) Pulmonary endothelial cell killing by human neutrophils. Possible involvement of hydroxyl radical. Lab Invest 53:656–663
Weiss SJ, LoBuglio AF (1982) Phagocyte-generated oxygen metabolites and cell injury. Lab Invest 47:5–18
Morishima I, Matsui H, Mukawa H, Hayashi K, Toki Y, Okumura K, Ito T, Hayakawa T (1998) Melatonin, a pineal hormone with antioxidant property, protects against adriamycin cardiomyopathy in rats. Life Sci 63:511–521
Zbinden G, Brändle E (1975) Toxicologic screening of daunorubicin (NSC-82151), adriamycin (NSC-123127), and their derivatives in rats. Cancer Chemother Rep 59:707–715
Sharma S, Stutzman JD, Kelloff GJ, Steele VE (1994) Screening of potential chemopreventive agents using biochemical markers of carcinogenesis. Cancer Res 54:5848–5855
Wattenberg LW (1985) Chemoprevention of cancer. Cancer Res 45:1–8
Devi Priya S, Shyamala Devi CS (1999) Protective effect of quercetin in cisplatin-induced cell injury in the rat kidney. Indian J Pharmacol 31:422–426
Chandrasekar MJN, Bommu P, Nanjan MJ, Suresh B (2006) Chemoprotective effect of Phyllanthus maderaspatensis in modulating cisplatin-induced nephrotoxicity and genotoxicity. Pharm Biol 44:100–106
Surh Y (1999) Molecular mechanisms of chemopreventive effects of selected dietary and medicinal phenolic substances. Mutat Res 428:305–327
Griffith OW (1999) Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic Biol Med 27:922–935
Acknowledgments
We thank Dr. Amit of Natural Remedies Pvt. Ltd., Bangalore, for providing the plant material. Praveen Bommu thanks the Department of Science and Technology, Govt. of India, New Delhi, India, for the award of a Senior Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bommu, P., Nanjan, C.M.J., Joghee, N.M. et al. Phyllanthus maderaspatensis, a dietary supplement for the amelioration of adriamycin-induced toxicity and oxidative stress in mice. J Nat Med 62, 149–154 (2008). https://doi.org/10.1007/s11418-007-0204-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-007-0204-1