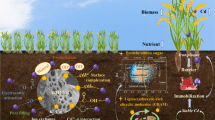
Abstract
Purpose
The competitive sorption of arsenite, As(III), and antimonite, Sb(III) on mackinawite (FeS) was investigated, so as to better understand the influence between As(III) and Sb(III) in anaerobic water, soil, or sediment systems rich in FeS.
Methods
FeS was synthesized and As(III) and Sb(III) were simultaneously or sequentially added into the FeS suspensions, so as to simulate the competitive sorption of As(III) and Sb(III) on the surface of FeS particles when As(III) and Sb(III) were parallelly sorbed or As(III) sorption was priorly aged.
Results
It was found that As(III) uptake by FeS could be significantly inhibited by Sb(III) at pH 7.0. When As(III) (initial concentration: 1 mg L−1) and Sb(III) were simultaneously added into FeS suspensions at pH 7.0, the presence of Sb(III) reduced the As(III) uptake by FeS from 51.8% (no Sb(III) added) to 22.7% (1 mg L−1 Sb(III) added) and to 6.9% (5 mg L−1 Sb(III) added), respectively. In contrast, As(III) uptake by FeS was only slightly inhibited at pH 5.5 and not inhibited at pH 9.0. It was postulated that the competitive sorption of As(III) and Sb(III) was primarily associated with the binding of As(III) and Sb(III) to FeS at the sulfur sites and the different chalcophility of Sb(III) and As(III) led to the significant replacement of As(III) by Sb(III) at pH 7.0. It was also found that aging of As(III) sorption significantly reduced the amount of As(III) that was outcompeted by Sb(III).
Conclusion
This study revealed the competitive sorption of As(III) and Sb(III) on FeS particles, and implicated the importance of competitive sorption in evaluating the mobilization or immobilization of arsenic or antimony in iron- and sulfur-rich anaerobic lake sediments or soils.




Similar content being viewed by others
Data availability
All data are included in the article.
References
Amarasiriwardena D, Wu F (2011) Antimony: emerging toxic contaminant in the environment. Microchem J 97:1–3
An X-L, Huang F-G, Ren H-T, Wang Y-F, Chen Y, Liu Z-M, Zhang H-W, Han X (2017) Oxidative dissolution of amorphous FeS and speciation of secondary Fe minerals: effects of pH and As(III) concentration. Chem Geol 462:44–54
APHA (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association/American Water Works Association, Baltimore, USA
Ashley PM, Craw D, Graham BP, Chappell DA (2003) Environmental mobility of antimony around mesothermal stibnite deposits, New South Wales, Australia and southern New Zealand. J Geochem Explor 77:1–14
Balsley S, Brady P, Krumhansl J, Anderson H (1996) Iodide retention by metal sulfide surfaces: cinnabar and chalcocite. Environ Sci Technol 30:3025–3027
Bebie J, Schoonen MAA, Fuhrmann M, Strongin DR (1998) Surface charge development on transition metal sulfides: an electrokinetic study. Geochim Cosmochim Acta 62:633–642
Biswas BK, Inoue J-I, Kawakita H, Ohto K, Inoue K (2009) Effective removal and recovery of antimony using metal-loaded saponified orange waste. J Hazard Mater 172:721–728
Burton ED, Johnston SG, Bush RT (2011) Microbial sulfidogenesis in ferrihydrite-rich environments: effects on iron mineralogy and arsenic mobility. Geochim Cosmochim Acta 75:3072–3087
Burton ED, Hockmann K, Karimian N, Johnston SG (2019) Antimony mobility in reducing environments: the effect of microbial iron(III)-reduction and associated secondary mineralization. Geochim Cosmochim Acta 245:278–289
Casiot C, Ujevic M, Munoz M, Seidel JL, Elbaz-Poulichet F (2007) Antimony and arsenic mobility in a creek draining an antimony mine abandoned 85 years ago. Appl Geochem 22:788–798
Ciardelli MC, Xu H, Sahai N (2008) Role of Fe(II), phosphate, silicate, sulfate, and carbonate in arsenic uptake by coprecipitation in synthetic and natural groundwater. Water Res 42:615–624
Coles CA, Rao SR, Yong R (2000) Lead and cadmium interactions with mackinawite: retention mechanisms and the role of pH. Environ Sci Technol 34:996–1000
Couture R-M, Rose J, Kumar N, Mitchell K, Wallschläger D, Cappellen PV (2013) Sorption of arsenite, arsenate, and thioarsenates to iron oxides and iron sulfides: a kinetic and spectroscopic investigation. Environ Sci Technol 47:5652–5659
Cui X, Ni Q, Lin Q, Khan KR, Li T, Khan MB, He Z, Yang X (2017) Simultaneous sorption and catalytic oxidation of trivalent antimony by Canna indica derived biochars. Environ Pollut 229:394–402
Dubey KP, Ghosh S (1962) Formation of thiosalt from antimonous sulfide. Zeit Anorg Chem 319:204–207
Eary LE (1992) The solubility of amorphous As2S3 from 25 to 90 °C. Geochim Cosmochim Acta 56:2267–2280
Emiroglu S, Rey D, Petersen N (2004) Magnetic properties of sediment in the Ria de Arousa (Spain): dissolution of iron oxides and formation of iron sulphides. Phys Chem Earth 29:947–959
Farquhar ML, Charnock JM, Livens FR, Vaughan DJ (2002) Mechanisms of arsenic uptake from aqueous solution by interaction with goethite, lepidocrocite, mackinawite, and pyrite: an X-ray absorption spectroscopy study. Environ Sci Technol 36(8):1757–1762
Fawcett SE, Jamieson HE, Nordstrom DK, McCleskey RB (2015) Arsenic and antimony geochemistry of mine wastes, associated waters and sediments at the Giant Mine, Yelllowknife, Northwest Territories, Canada. Appl Geochem 62:3–17
Filella M, Belzile N, Chen Y-W (2002) Antimony in the environment: a review focused on natural waters II. Relevant solution chemistry. Earth Sci Rev 59:265–285
Gallegos TJ, Han Y-S, Hayes KF (2008) Model predictions of realgar precipitation by reaction of As(III) with synthetic mackinawite under anoxic conditions. Environ Sci Technol 42:9338–9343
Gallegos TJ, Hyun SP, Hayes KF (2007) Spectroscopic investigation of the uptake of arsenite from solution by synthetic mackinawite. Environ Sci Technol 41:7781–7786
Griggs CS, Martin WA, Larson SL, O’Connnor G, Fabian G, Zynda G, Mackie D (2011) The effect of phosphate application on the mobility of antimony in firing range soils. Sci Total Environ 409:2397–2403
Han Y-S, Demond AH, Hayes KF (2013) Impact of dissolved silica on arsenite removal by nano-particulate FeS and FeS-coated sand. Chemosphere 92:477–481
Han Y-S, Gallegos TJ, Demond AH, Hays KF (2011a) FeS-coated sand for removal of arsenic(III) under anaerobic conditions in permeable reactive barriers. Water Res 45:593–604
Han Y-S, Jeong HY, Demond AH, Hayes KF (2011b) X-ray absorption and photoelectron spectroscopic study of the association of As(III) with nanoparticulate FeS and FeS-coated sand. Water Res 45:5727–5735
Han Y-S, Park JH, Min Y, Lim D-H (2020) Competitive adsorption between phosphate and arsenic in soil containing iron sulfide: XAS experiment and DFT calculation approaches. Chem Eng J 397:125426
Han Y-S, Seong HJ, Chon C-M, Park JH, Nam I-H, Yoo K, Ahn JS (2018) Interaction of Sb(III) with iron sulfide under anoxic conditions: similarities and differences compared to As(III) interactions. Chemosphere 195:762–770
Jeong HY, Klaue B, Blum JD, Hayes KF (2007) Sorption of mercuric ion by synthetic nanocrystalline mackinawite (FeS). Environ Sci Technol 41:7699–7705
Jong T, Parry DL (2003) Removal of sulfate and heavy metals by sulfate reducing bacteria in short-term bench scale upflow anaerobic packed bed reactor runs. Water Res 37:3379–3389
Kirsch R, Scheinost AC, Rossberg A, Banerjee D, Charlet L (2008) Reduction of antimony by nano-particulate magnetite and mackinawite. Mineral Mag 72:185–189
Kolbe F, Weiss H, Morgenstern P, Wennrich R, Lorenz W, Schurk K, Stanjek H, Daus B (2011) Sorption of aqueous antimony and arsenic species onto akaganeite. J Colloid Interf Sci 357:460–465
Krupka KM, Serne RJ (2002) Geochemical factors affecting the behavior of antimony, cobalt, europium, technetium, and uranium in vadose sediments. Pacific Northwest National Laboratory, Washington
Krupp RE (1988) Solubility of stibnite in hydrogen-sulfide solutions, speciation, and equilibrium-constants, from 25 to 350 °C. Geochim Cosmochim Acta 52:3005–3015
Kulp TR, Miller LG, Braiotta F, Webb SM, Kocar BD, Blum JS, Oremland RS (2014) Microbiological reduction of Sb(V) in anoxic freshwater sediments. Environ Sci Technol 48:218–226
Li D, Zhang G, Wang Q, Liu S, Ma C, Chen J, Liu F (2021) Interaction of aqueous antimony(III) with synthetic ferrous sulfide. Appl Geochem 128:104957
Luther S, Borgfeld N, Kim J, Parsons JG (2012) Removal of arsenic from aqueous solution: a study of the effects of pH and interfering ions using iron oxide nanomaterials. Microchem J 101:30–36
Ma C, Zhang G, Chen J, Wang Q, Liu F (2020) Transfer of FeS-bound arsenic into pyrite during the transformation of amorphous FeS to pyrite. Appl Geochem 119:104645
Mane RS, Lokhande CD (2003) Thickness-dependent properties of chemically deposited Sb2S3 thin films. Mater Chem Phys 82:347–354
Marzi M, Towfighi H, Shahbazi K, Farahbakhsh M, Kazemian H (2022) Study of arsenic adsorption in calcareous soils: competitive effect of phosphate, citrate, oxalate, humic acid and fulvic acid. J Environ Manage 318:115532
Mok W-M, Wai CM (1990) Distribution and mobilization of arsenic and antimony species in the Coeur D’Alene river, Idaho. Environ Sci Technol 24:102–108
Morse JW, Millero FJ, Cornwell JC, Rickard D (1987) The chemistry of the hydrogen sulfide and iron sulfide systems in natural waters. Earth Sci Rev 24:1–42
Niazi NK, Burton ED (2016) Arsenic sorption to nanoparticulate mackinawite (FeS): an examination of phosphate competition. Environ Pollut 218:111–117
Olsen NJ, Mountain BW, Seward TM (2018) Antimony(III) sulfide complexes in aqueous solutions at 30 °C: a solubility and XAS study. Chem Geol 476:233–247
Qi P, Pichler T (2016) Sequential and simultaneous adsorption of Sb(III) and Sb(V) on ferrihydrite: implications for oxidation and competition. Chemosphere 145:55–60
Qi P, Pichler T (2017) Competitive adsorption of As(III), As(V), Sb(III) and Sb(V) onto ferrihydrite in multi-component systems: implications for mobility and distribution. J Hazard Mater 330:142–148
Rickard D (2006) The solubility of FeS. Geochem Cosmochim Acta 70:5779–5789
Rodriguez-Freire L, Sierra-Alvarez R, Root R, Chorover J, Field J (2014) Biomineralization of arsenate to arsenic sulfides is greatly enhanced at mildly acidic conditions. Water Res 66:242–253
Vega AS, Planer-Friedrich B, Pasten PA (2017) Arsenite and arsenate immobilization by preformed and concurrently formed disordered mackinawite (FeS). Chem Geol 475:62–75
Wang Q, Zhang G, Liu S, Mao K, Ma C, Chen J (2022) The impact of phosphate on the interaction of Sb(III) with ferrous sulfide. Appl Geochem 140:105297
Wilkin R, Ford R (2002) Use of hydrochloric acid for determining solid-phase arsenic partitioning in sulfidic system. Environ Sci Technol 36:4921–4927
Wilson SC, Lockwood PV, Ashley PM, Tighe M (2010) The chemistry and behaviour of antimony in the soil environment with comparisons to arsenic: a critical review. Environ Pollut 158:1169–1181
Wolthers M, Charlet L, van der Weijden CH, van der Linde PR, Ricard D (2005) Arsenic mobility in the ambient sulfidic environment; sorption of arsenic(V) and arsenic(III) onto disordered mackinawite. Geochim Cosmochim Acta 69:3483–3492
Wolthers M, van der Gaast SJ, Rickard D (2003) The structure of disordered mackinawite. Am Mineral 88:2007–2015
Zhang G, Liu H, Liu R, Qu J (2009) Adsorption behavior and mechanism of arsenate at Fe–Mn binary oxide/water interface. J Hazard Mater 168:820–825
Funding
This study was financially supported by the China National Key Research and Development Program (No. 2020YFC1807700) and the China National Natural Science Foundation (No. U1612442).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Responsible editor: Sophie Ayrault
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• As(III) sorption on FeS was inhibited by Sb(III).
• The competitive sorption of As(III) and Sb(III) was strongest at neutral pH.
• Aging of As(III) sorption weakened the competitive effect of Sb(III).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Q., Zhang, G., Ma, C. et al. Competitive sorption of arsenic and antimony onto synthetic ferrous sulfide. J Soils Sediments (2024). https://doi.org/10.1007/s11368-024-03791-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11368-024-03791-0