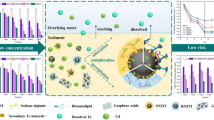
Abstract
Purpose
Currently, cadmium (Cd) is extensively present in sediments and is more mobile and toxic than other heavy metals. Sulfidized nanoscale zero-valent iron (S-nZVI) has been widely applied for the removal of heavy metals due to its unique core–shell structure. However, its remediation effect on Cd in sediments is still unclear. In addition, the precursors used in the synthesis also affect the reactivity of S-nZVI. Therefore, in this research, we evaluated the impacts of different iron precursors (Fe2+ and Fe3+) on the properties of nanoscale zero-valent iron (nZVI) and S-nZVI, and investigated the mechanism of S-nZVI in the immobilization of Cd-contaminated sediments.
Materials and methods
Synthesized nanomaterials were added to Cd-contaminated sediments. The Cd speciation was investigated using the modified Community Bureau of Reference (BCR) sequential extraction method. To evaluate the immobilization efficiency by different nanomaterials, toxicity characteristic leaching procedure (TCLP)–leachable and diethylene triamine pentaacetic acid (DTPA)–extractable Cd were measured. The changes in sediment properties and enzyme activity, as well as the mechanism of Cd immobilization by S-nZVI, were also examined.
Results and discussion
The BCR extraction results indicated that synthesized nanomaterials effectively transformed the acid soluble Cd into residual speciation. In comparison to the control group, the TCLP-leachable Cd was decreased by 99.36% and 98.96% in the S-nZVI (Fe2+) and S-nZVI (Fe3+) treated groups, respectively, while the DTPA-extractable Cd decreased by 95.31% and 94.3%, respectively, after 15 days of incubation. The sediment physicochemical properties demonstrated that the change of pH and Eh were crucial factors for Cd immobilization. Significant enhancement of urease and sucrase activity was observed. After remediation, characterization analyses of S-nZVI (Fe2+) showed that Cd was successfully enriched, with CdS, CdO and CdFe2O4 being the main forms.
Conclusion
S-nZVI has a superior remediation potential for Cd contamination in sediments. Compared with others, S-nZVI (Fe2+) was the most effective for Cd immobilization. Hence, the work offers new insights into the selection of iron precursors for S-nZVI nanomaterials and their practical applications in the remediation of heavy metal contaminated sediments.










Similar content being viewed by others
Data availability
Data are available upon request.
References
Akcay H, Oguz A, Karapire C (2003) Study of heavy metal pollution and speciation in Buyak Menderes and Gediz river sediments. Water Res 37(4):813–822. https://doi.org/10.1016/S0043-1354(02)00392-5
Cheng YJ, Dong HR, Lu Y, Hou KJ, Wang YY, Ning Q, Li L, Wang B, Zhang LH, Zeng GM (2019) Toxicity of sulfide-modified nanoscale zero-valent iron to Escherichia coli in aqueous solutions. Chemosphere 220:523–530. https://doi.org/10.1016/j.chemosphere.2018.12.159
Djebbi MA, Allagui L, El-Ayachi MS, Boubakri S, Jaffrezic-Renault N, Namour P, Amara AB (2022) Zero-valent iron nanoparticles supported on biomass-derived porous carbon for simultaneous detection of Cd2+ and Pb2+. Acs Appl Nano Mater 5(1):546–558. https://doi.org/10.1021/acsanm.1c03333
Fu RB, Yang YP, Xu Z, Zhang X, Guo XP, Bi DS (2015) The removal of chromium (VI) and lead (II) from groundwater using sepiolite-supported nanoscale zero-valent iron (S-NZVI). Chemosphere 138:726–734. https://doi.org/10.1016/j.chemosphere. 2015.07.051
Gao F, Ahmad S, Tang J, Zhang C, Li S, Yu C, Liu Q, Sun H (2022a) Enhanced nitrobenzene removal in soil by biochar supported sulfidated nano zerovalent iron: solubilization effect and mechanism. Sci Total Environ 826:153960. https://doi.org/10.1016/j.scitotenv.2022.153960
Gao RQ, Hu PW, Dai YN, Zhang Y, Liu L, Yang WZ (2022b) Removal of cadmium(II) from aqueous solutions by a novel sulfide-modified nanoscale zero-valent iron supported on kaolinite: treatment efficiency, kinetics and mechanisms. Appl Surf Sci 602:154353. https://doi.org/10.1016/j.apsusc.2022.154353
Gotic M, Music S (2007) Mossbauer, FT-IR and FE SEM investigation of iron oxides precipitated from FeSO4 solutions. J Mol Struct 834:445–453. https://doi.org/10.1016/j.molstruc.2006.10.059
Grybos M, Davranche M, Gruau G, Petitjean P, Pedrot M (2009) Increasing pH drives organic matter solubilization from wetland soils under reducing conditions. Geoderma 154:13–19. https://doi.org/10.1016/j.geoderma.2009.09.001
Guan XH, Sun YK, Qin HJ, Li JX, Lo IMC, He D, Dong HR (2015) The limitations of applying zero-valent iron technology in contaminants sequestration and the corresponding countermeasures: the development in zero-valent iron technology in the last two decades (1994–2014). Water Res 75:224–248. https://doi.org/10.1016/j.watres.2015.02.034
Guan XH, Yang HY, Sun YK, Qiao JL (2019) Enhanced immobilization of chromium(VI) in soil using sulfidated zero-valent iron. Chemosphere 228:370–376. https://doi.org/10.1016/j.chemosphere.2019.04.132
Guo YQ, Li XQ, Liang L, Lin Z, Su XT, Zhang WC (2021) Immobilization of cadmium in contaminated soils using sulfidated nanoscale zero-valent iron: effectiveness and remediation mechanism. J Hazard Mater 420:126605. https://doi.org/10.1016/j.jhazmat.2021.126605
He F, Li ZJ, Shi SS, Xu WQ, Sheng HZ, Gu YW, Jiang YH, Xi BD (2018) Dechlorination of excess trichloroethene by bimetallic and sulfidated nanoscale zero-valent iron. Environ Sci Technol 52(15):8627–8637. https://doi.org/10.1021/acs.est.8b01735
He YH, Fang TT, Wang J, Liu XY, Yan ZG, Lin H, Li FS, Guo GL (2022) Insight into the stabilization mechanism and long-term effect on As, Cd, and Pb in soil using zeolite-supported nanoscale zero-valent iron. J Clean Prod 355:131634. https://doi.org/10.1016/j.jclepro.2022.131634
Huang DL, Hu ZX, Peng ZW, Zeng GM, Chen GM, Zhang C, Cheng M, Wan J, Wang X, Qin X (2018) Cadmium immobilization in river sediments using stabilized nanoscale zero-valent iron with enhanced transport by polysaccharide coating. J Environ Manage 210:191–200. https://doi.org/10.1016/j.jenvman.2018.01.001
Ibrar A, Kazmi M, Khan A, Halim SA, Saeed A, Mehsud S, Al-Harrasi A, Khan I (2020) Robust therapeutic potential of carbazole-triazine hybrids as a new class of urease inhibitors: a distinctive combination of nitrogen-containing heterocycles. Bioorganic Chem 95:103479. https://doi.org/10.1016/j.bioorg.2019.103479
Kim EJ, Kim JH, Azad AM, Chang YS (2011) Facile synthesis and characterization of Fe/FeS nanoparticles for environmental applications. ACS Appl Mater Interfaces 3(5):1457–1462. https://doi.org/10.1021/am200016v
Li Q, Guo BD, Yu JG, Ran JR, Zhang BH, Yan HJ, Gong JR (2011) Highly efficient visible-light-driven photocatalytic hydrogen production of CdS-cluster-decorated graphene nanosheets. J Am Chem Soc 133(28):10878–10884. https://doi.org/10.1021/ja2025454
Li XC, Yang ZZ, Zhang C, Wei JJ, Zhang HQ, Li ZH, Ma C, Wang MS, Chen JQ, Hu JW (2019) Effects of different crystalline iron oxides on immobilization and bioavailability of Cd in contaminated sediments. Chem Eng J 373:307–317. https://doi.org/10.1016/j.cej.2019.05.015
Liang L, Li XQ, Lin Z, Tian C, Guo YQ (2020) The removal of Cd by sulfidated nanoscale zero-valent iron: the structural, chemical bonding evolution and the reaction kinetics. Chem Eng J 382:122933. https://doi.org/10.1016/j.cej.2019.122933
Liu MJ, Xu M, Zhang X, Zhou JJ, Ma QX, Wu LX (2021a) Poorly crystalline Fe(II) mineral phases induced by nano zero-valent iron are responsible for Cd stabilization with different soil moisture conditions and soil types. Ecotox Environ Safe 223:112616. https://doi.org/10.1016/j.ecoenv.2021.112616
Liu QQ, Sheng YQ, Wang WJ, Li CY, Zhao GQ (2020a) Remediation and its biological responses of Cd contaminated sediments using biochar and minerals with nanoscale zero-valent iron loading. Sci Total Environ 713:136650. https://doi.org/10.1016/j.scitotenv.2020.136650
Liu SJ, Liu YG, Tan XF, Zeng GM, Zhou YH, Liu SB, Yin ZH, Jiang LH, Li MF, Wen J (2018) The effect of several activated biochars on Cd immobilization and microbial community composition during in-situ remediation of heavy metal contaminated sediments. Chemosphere 208:655–664. https://doi.org/10.1016/j.chemosphere.2018.06.023
Liu YL, Huang YD, Zhang C, Li WY, Chen CY, Zhang Z, Chen HY, Wang JJ, Li YT, Zhang YL (2020b) Nano-FeS incorporated into stable lignin hydrogel: a novel strategy for cadmium removal from soil. Environ Pollut 264:114739. https://doi.org/10.1016/j.envpol.2020.114739
Liu YZ, Wu T, White JC, Lin DH (2021) A new strategy using nanoscale zero-valent iron to simultaneously promote remediation and safe crop production in contaminated soil. Nat Nanotechnol 16(2):197–205. https://doi.org/10.1038/s41565-020-00803-1
Lv D, Zhou JS, Cao Z, Xu J, Liu YL, Li YZ, Yang KL, Lou ZM, Lou LP, Xu XH (2019) Mechanism and influence factors of chromium (VI) removal by sulfide-modified nanoscale zerovalent iron. Chemosphere 224:306–315. https://doi.org/10.1016/j.chemosphere.2019.02.109
Lv D, Zhou XX, Zhou JS, Liu YL, Li YZ, Yang KL, Lou ZM, Baig SA, Wu DL, Xu XH (2018) Design and characterization of sulfide-modified nanoscale zerovalent iron for cadmium(II) removal from aqueous solutions. Appl Surf Sci 442:114–123. https://doi.org/10.1016/j.apsusc.2018.02.085
Mandal S, Pu SY, Wang XK, Ma H, Bai YC (2020) Hierarchical porous structured polysulfide supported nZVI/biochar and efficient immobilization of selenium in the soil. Sci Total Environ 708:134831. https://doi.org/10.1016/j.scitotenv.2019.134831
Shen GQ, Lu YT, Zhou QX, Hong JB (2005) Interaction of polycyclic aromatic hydrocarbons and heavy metals on soil enzyme. Chemosphere 61(8):1175–1182. https://doi.org/10.1016/j.chemosphere.2005.02.074
Song HH, Liang WY, Luo KL, Wang GH, Li QN, Ji XW, Wan J, Shao XC, Gong KL, Zhang W, Peng C (2023) Simultaneous stabilization of Pb, Cd, and As in soil by rhamnolipid coated sulfidated nano zero-valent iron: effects and mechanisms. J Hazard Mater 443:130259. https://doi.org/10.1016/j.jhazmat.2022.130259
Song SK, Su MM, Adeleye AS, Zhang YL, Zhou XF (2017) Optimal design and characterization of sulfide-modified nanoscale zerovalent iron for diclofenac removal. Appl Catal B-Environ 201:211–220. https://doi.org/10.1016/j.apcatb.2016.07.055
Sun YB, Sun GH, Xu YM, Wang L, Lin DS, Liang XF, Shi X (2012) In situ stabilization remediation of cadmium contaminated soils of wastewater irrigation region using sepiolite. J Environ Sci 24(10):1799–1805. https://doi.org/10.1016/S1001-0742(11)61010-3
Tang JC, Zhao BB, Lyu HH, Li D (2021) Development of a novel pyrite/biochar composite (BM-FeS2@BC) by ball milling for aqueous Cr(VI) removal and its mechanisms. J Hazard Mater 413:125415. https://doi.org/10.1016/j.jhazmat.2021.125415
US EPA (1992) Toxicity characteristic leaching procedure (No. Method 1311) in SW-846. Office of Solid Waste, Washington DC
Wang YL, Lin DH (2017) The interaction between nano zero-valent iron and soil components and its environmental implication. Prog Chem 29(9):1072–1081. https://doi.org/10.7536/PC170526
Wen J, Yi YJ, Zeng GM (2016) Effects of modified zeolite on the removal and stabilization of heavy metals in contaminated lake sediments using BCR sequential extraction. J Environ Manage 178:63–69. https://doi.org/10.1016/j.jenvman.2016.04.046
Wu YH, Pang HW, Liu Y, Wang XX, Yu SJ, Fu D, Chen JR, Wang XK (2019) Environmental remediation of heavy metal ions by novel-nanomaterials: a review. Environ Pollut 246:608–620. https://doi.org/10.1016/j.envpol.2018.12.076
Xiao L, Li MH, Dai J, Motelica-Heino M, Chen XF, Wu JL, Zhao LF, Liu KX, Zhang C (2020) Assessment of earthworm activity on Cu, Cd, Pb and Zn bioavailability in contaminated soils using biota to soil accumulation factor and DTPA extraction. Ecotox Environ Safe 195:110513. https://doi.org/10.1016/j.ecoenv.2020.110513
Xiao SJ, Jin ZL, Dong HR, Xiao JY, Li YJ, Li L, Li R, Chen J, Tian R, Xie QQ (2022) A comparative study on the physicochemical properties, reactivity and long-term performance of sulfidized nanoscale zerovalent iron synthesized with different kinds of sulfur precursors and procedures in simulated groundwater. Water Res 212:118097. https://doi.org/10.1016/j.watres.2022.118097
Xu BD, Qian TT, Chen S, Yang J, Jiang H (2021) Preparation of highly stable and easily regenerated sulfuretted nZVI via one-pot fast pyrolysis method for the removal of diclofenac. J Environ Chem Eng 9(4):105425. https://doi.org/10.1016/j.jece.2021.105425
Xu J, Avellan A, Li H, Clark EA, Henkelman G, Kaegi R, Lowry GV (2020a) Iron and sulfur precursors affect crystalline structure, speciation, and reactivity of sulfidized nanoscale zerovalent iron. Environ Sci Technol 54(20):13294–13303. https://doi.org/10.1021/acs.est.0c03879
Xu J, Avellan A, Li H, Liu XT, Noel V, Lou ZM, Wang Y, Kaegi R, Henkelman G, Lowry GV (2020b) Sulfur loading and speciation control the hydrophobicity, electron transfer, reactivity, and selectivity of sulfidized nanoscale zerovalent iron. Adv Mater 32(17):1906910. https://doi.org/10.1002/adma.201906910
Xue WJ, Cao S, Zhu J, Li WY, Li J, Huang DL, Wang RZ, Gao Y (2022) Stabilization of cadmium in contaminated sediments based on a nanoremediation strategy: environmental impacts and mechanisms. Chemosphere 287:132363. https://doi.org/10.1016/j.chemosphere.2021.132363
Yang B, Cao YZ, Ren J, Wang M, Luo HL, Li FS (2019) Water incubation-induced fluctuating release of heavy metals in two smelter-contaminated soils. J Environ Sci 82:14–23. https://doi.org/10.1016/j.jes.2019.02.026
Yang D, Yang SY, Wang L, Xu JM, Liu XM (2021a) Performance of biochar-supported nanoscale zero-valent iron for cadmium and arsenic co-contaminated soil remediation: insights on availability, bioaccumulation and health risk. Environ Pollut 290:118054. https://doi.org/10.1016/j.envpol.2021.118054
Yang D, Yang SY, Yuan HH, Wang F, Wang HL, Xu JM, Liu XM (2021b) Co-benefits of biochar-supported nanoscale zero-valent iron in simultaneously stabilizing soil heavy metals and reducing their bioaccessibility. J Hazard Mater 418:126292. https://doi.org/10.1016/j.jhazmat.2021.126292
Yang R, Xia XM, Wang JH, Zhu LS, Wang J, Ahmad Z, Yang LL, Mao SS, Chen YY (2020) Dose and time-dependent response of single and combined artificial contamination of sulfamethazine and copper on soil enzymatic activities. Chemosphere 250:126161. https://doi.org/10.1016/j.chemosphere.2020.126161
Zhang C, Lu J, Wu J (2020) One-step green preparation of magnetic seaweed biochar/sulfidated Fe 0 composite with strengthen adsorptive removal of tetrabromobisphenol A through in situ reduction. Bioresour Technol 307:123170. https://doi.org/10.1016/j.biortech.2020.123170
Zhang XW, Liu XD, Peng Y, Wu XY, Tan YJ, Zeng Q, Song ZJ, Li M (2022a) Controllable shell corrosion of coated nanoscale zero valent iron induces long-term potentiation of its reactivity for uranium removal. Sep Purif Technol 287:120550. https://doi.org/10.1016/j.seppur.2022.120550
Zhang Y, Wu CF, Deng SP, Zhang JL, Hou JY, Wang C, Fu ZC (2022b) Effect of different washing solutions on soil enzyme activity and microbial community in agricultural soil severely contaminated with cadmium. Environ Sci Pollut Res 29(36):54641–54651. https://doi.org/10.1007/s11356-022-19734-6
Zhang YY, Jiang H, Zhang Y, Xie JF (2013) The dispersity-dependent interaction between montmorillonite supported nZVI and Cr(VI) in aqueous solution. Chem Eng J 229:412–419. https://doi.org/10.1016/j.cej.2013.06.031
Funding
This work was financially supported by the Program for the National Natural Science Foundation of China (42107415) and Natural Science Foundation of Jiangsu Province (BK20210830).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Responsible editor: Patrick Byrne
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xue, W., Wen, S., Zhu, Y. et al. Immobilization of cadmium in river sediments using sulfidized nanoscale zero-valent iron synthesized with different iron precursors: performance and mechanism. J Soils Sediments 23, 3550–3566 (2023). https://doi.org/10.1007/s11368-023-03606-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-023-03606-8