Abstract
Purpose
Oxalic acid–activated phosphate rock (APR) can efficiently immobilize Pb in soil and water. However, the application of APR could cause acidification in environment. This study aims to investigate the joint effects of APR and kaolinite on the Pb(II) removal in simulated wastewater.
Materials and methods
A series of experiments combined with X-ray diffraction (XRD) analysis was conducted to investigate the joint effects of APR and kaolinite on Pb removal, P concentration, and acidity.
Results and discussion
The results indicated that the data of isothermal adsorption of Pb by kaolinite was well-fitted with the Langmuir adsorption isotherm equation, while the Pb removal rates using APR and the mixture of kaolinite and APR (kaolinite + APR) increased with the increasing initial Pb concentration ranging from 0 to 400 mg/L. The Pb removal rates with kaolinite decreased from 51.85 to 6.91% and increased from 97.15 to 99.35% with APR. With kaolinite + APR, the rates changed between 81.74 and 98.18%.
Conclusion
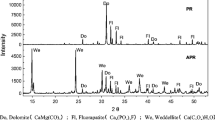
The X-ray diffraction patterns and the various desorption results with two different desorption agents further indicated that the removal of Pb(II) using kaolinite is an adsorption process, while Pb phosphate precipitates are formed during the removal of Pb(II) both with APR and kaolinite + APR. There are two advantages with the application of kaolinite + APR in Pb removal: (i) Compared to the use of kaolinite only, Pb is bound tightly in kaolinite + APR treatment with a slight decrease of solution pH; (ii) phosphorus effectiveness is still great in an equilibrium solution. This study serves as a scientific evidence for the comprehensive utilization of kaolinite + APR in the removal of Pb from water and soil.






Similar content being viewed by others
References
Adebowale KO, Unuabonah IE, Olu-Owolabi BI (2006) The effect of some operating variables on the adsorption of lead and cadmium ions on kaolinite clay. J Hazard Mater 134:130–139
Antelo J, Fiol S, Perez C, Marino S, Arce F, Gondar D, Lopez R (2010) Analysis of phosphate adsorption onto ferrihydrite using the CD-MUSIC model. J Colloid Interface Sci 347:112–119
Bao SD (2000) Agrochemical analysis of soil. China Agriculture Press, Beijing, China
Bhattacharyya KG, Gupta SS (2008) Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: a review. Adv Colloid Interfac 140:114–131
Cao XD, Wahbi A, Ma L, Li B, Yang YL (2009) Immobilization of Zn, Cu, and Pb in contaminated soils using phosphate rock and phosphoric acid. J Hazard Mater 164:555–564
Chang JH, Dong CD, Huang SH, Shen SY (2020) The study on lead desorption from the real-field contaminated soil by circulation-enhanced electrokinetics (CEEK) with EDTA. J Hazard Mater 383:1–6
Dai YH, Liang Y, Xu XY, Zhao L, Cao XD (2017) An integrated approach for simultaneous immobilization of lead in both contaminated soil and groundwater: laboratory test and numerical modeling. J Hazard Mater 342:107–113
Elouear Z, Bouzid J, Boujelben N, Feki M, Jamoussic F, Montield A (2008) Heavy metal removal from aqueous solutions by activated phosphate rock. J Hazard Mater 156:412–420
Fayiga A, Nwoke C (2016) Phosphate rock: origin, importance, environmental impacts and future roles. Environ Rev 24:403–415
Gupta SS, Bhattacharyya KG (2008) Immobilization of Pb(II), Cd(II) and Ni(II) ions on kaolinite and montmorillonite surfaces from aqueous medium. J Environ Manage 87:46–58
Gupta SS, Bhattacharyya KG (2012) Adsorption of heavy metals on kaolinite and montmorillonite: a review. Phys Chem Chem Phys 14:6698–6723
He M, Shi H, Zhao XY, Yu Y, Qu B (2013) Immobilization of Pb and Cd in contaminated soil using nano-crystallite hydroxyapatite. Procedia Environ Sci 18:657–665
He Y, Xia DY, Xin J, Sun T (2006) A study on the kinetics of lead ion adsorption to kaolinite. Tech Equip Environ Poll Control 7:82–85
Hou DY, O’Connor D, Nathanail P, Tian L, Ma Y (2017) Integrated GIS and multivariate statistical analysis for regional scale assessment of heavy metal soil contamination: a critical review. Environ Pollut 231:1188–1200
Hu XN, Nan ZR, Wang SL, Huang H, Hu ZY (2009) Sorption and desorption of copper, zinc and lead in the irrigated desert soil from the oasis in the arid regions, northwest China. Ecol Environ Sci 18:2183–2188
Huang GY, Gao RL, You JW, Zhu J, Fu QL, Hu HQ (2019) Oxalic acid activated phosphate rock and bone meal to immobilize Cu and Pb in mine soils. Ecotox Environ Safe 174:401–407
Huang GY, Guo GG, Yao SY, Zhang N, Hu HQ (2016a) Organic acids, amino acids compositions in the root exudates and Cu-accumulation in castor (Ricinus communis L.) under Cu stress. Int J Phytoremediat 18(1):33–40
Huang GY, Su XJ, Rizwan MS, Zhu Y, Hu HQ (2016b) Chemical immobilization of Pb, Cu, and Cd by phosphate materials and calcium carbonate in contaminated soils. Environ Sci Pollut R 23:16845–16856
Jiang GJ, Liu YH, Huang L, Fu QL, Deng YJ, Hu HQ (2012) Mechanism of lead immobilization by oxalic acid-activated phosphate rocks. J Environ Sci 24:919–925
Jing MQ, Jin XY, Lu XQ, Chen ZL (2010) Adsorption of Pb(II), Cd(II), Ni(II) and Cu(II) onto natural kaolinite clay. Desalination 252:33–39
Kamiyango MW, Masamba WRL, Sajidu SMI, Fabiano E (2009) Phosphate removal from aqueous solutions using kaolinite obtained from Linthipe, Malawi. Phys Chem Earth 34:850–856
Khan MS, Zaidi A, Wani PA (2007) Role of phosphate-solubilizing microorganisms in sustainable agriculture - a review. Agron Sustain Dev 27:29–43
Li LP, Zhao Q, Zhang HY, Scheckel KG (2017) Enhancement of phosphate immobilization of Pb in contaminated soils with Ca and Cl amendment. Acta Sci Circumst 37:4344–4351
Liu XF, Hicher P, Muresan B, Saiyouri N, Hicher PY (2016) Heavy metal retention properties of kaolin and bentonite in a wide range of concentration and different pH conditions. Appl Clay Sci 119:365–374
Liu Y H, Hu HQ, Jiang GJ, Fu QL (2009) A method of making phosphate fertilizer using phosphate rocks activated by organic acid. China, CN1010659569
Lu RK (1999) Agriculture chemical analysis methods of soil. China Agricultural Science and Technology Press, Beijing, China
Ma QY, Logan TJ, Traina SJ (1995) Lead immobilization from aqueous solutions and contaminated soils using phosphate rocks. Environ Sci Technol 29:1118–1126
Mander C, Wakelin S, Young S, Condron L, O’Callaghan M (2012) Incidence and diversity of phosphate-solubilising bacteria are linked to phosphorus status in grassland soils. Soil Biol Biochem 44:93–101
Mbey JA, Thomas F, Razafitianamaharavo A, Caillet C, Villiéras F (2019) A comparative study of some kaolinites surface properties. Appl Clay Sci 172:135–145
Melamed R, Cao XD, Chen M, Ma LQ (2003) Field assessment of lead immobilization in a contaminated soil after phosphate application. Sci Total Environ 305:117–127
Moharami S, Jalali M (2013) Removal of phosphorus from aqueous solution by Iranian natural adsorbents. Chem Eng J 223:328–339
Muhammad D, Chen F, Zhao J, Zhang G, Wu F (2009) Comparison of EDTA- and citric acid-enhanced phytoextraction of heavy metals in artificially metal contaminated soil by Typha angustifolia. Int J Phytoremediat 11:558–574
Otunola BO, Ololade OO (2020) A review on the application of clay minerals as heavy metal adsorbents for remediation purposes. Environ Technol Inno 18:1–14
Park JH, Bolan N, Megharaj M (2011) Isolation of phosphate solubilizing bacteria and their potential for lead immobilization in soil. J Hazard Mater 185:829–836
Peen CJ, Warren JG (2009) Investigating phosphorus sorption onto kaolinite using isothermal titration calorimetry. Soil Sci Soc Am J 73:560–568
Sari A, Tuzen M, Citak D, Soylak M (2007) Equilibrium, kinetic and thermodynamic studies of adsorption of Pb(II) from aqueous solution onto Turkish kaolinite clay. J Hazard Mater 149:283–291
Srivastava P, Singh B, Angove M (2005) Competitive adsorption behavior of heavy metals on kaolinite. J Colloid Interf Sci 290:28–38
Su XJ, Zhu J, Zuo FuQL, JC, Liu YH, Hu HQ, (2015) Immobilization of lead in anthropogenic contaminated soils using phosphates with/without oxalic acid. J Environ Sci 28:64–73
Teng ZD, Shao W, Zhang KY, Huo YQ, Li M (2019) Characterization of phosphate solubilizing bacteria isolated from heavy metal contaminated soils and their potential for lead immobilization. J Environ Manage 231:189–197
Unuabonah EI, Adebowale KO, Olu-Owolabi BI (2007) Kinetic and thermodynamic studies of the adsorption of lead(II) ions onto phosphate-modified kaolinite clay. J Hazard Mater 144:386–395
Unuabonah EI, Olu-Owolabi BI, Okoroc D, Adebowale KO (2009) Comparison of two-stage sorption design models for the removal of lead ions by polyvinyl-modified kaolinite clay. J Hazard Mater 171:215–221
Valipour M, Shahbazi K, Khanmirzaei A (2016) Chemical immobilization of lead, cadmium, copper, and nickel in contaminated soils by phosphate amendments. Clean : Soil, Air, Water 44:572–578
Wei W, Cui J, Wei ZG (2014) Effects of low molecular weight organic acids on the immobilization of aqueous Pb(II) using phosphate rock and different crystallized hydroxyapatite. Chemosphere 105:14–23
Wei YQ, Zhao Y, Shi MZ, Cao ZY, Lu Q, Yang TX, Fan YY, Wei ZM (2018) Effect of organic acids production and bacterial community on the possible mechanism of phosphorus solubilization during composting with enriched phosphate-solubilizing bacteria inoculation. Bioresource Technol 247:190–199
Xia WY, Feng YS, Jin F, Zhang LM, Du YJ (2017) Stabilization and solidification of a heavy metal contaminated site soil using a hydroxyapatite based binder. Constr Build Mater 156:199–207
Xiong Y (1983) Material basis of soil colloid. Science Press, Beijing, China
Yadava VB, Gadia R, Kalrab S (2019) Clay based nanocomposites for removal of heavy metals from water: a review. J Environ Manage 232:803–817
Zeng GM, Wan J, Huang DL, Hu L, Huang C, Cheng M, Xue WJ, Gong XM, Wang RZ, Jiang DN (2017) Precipitation, adsorption and rhizosphere effect: the mechanisms for Phosphate-induced Pb immobilization in soils-a review. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2017.05.038
Zhang W, Huang H, Tan F, Wang H, Qiu R (2010) Influence of EDTA washing on the species and mobility of heavy metals residual in soils. J Hazard Mater 173:369–376
Zhao FJ, Ma YB, Zhu YG, Tang Z, McGrath SP (2015) Soil contamination in China: current status and mitigation strategies. Environ Sci Technol 49:750–759
Acknowledgements
The authors gratefully acknowledge the English teacher of Huazhen Wen for her language refinement of the manuscript.
Funding
The research was financially supported by the National Natural Science Foundations of China (Grants 42067030), the Youth Fund Project of Science and Technology Department of Jiangxi Province (20161BAB213081), and the Science and Technology Research Project of Education Department of Jiangxi Province (GJJ180231).
Author information
Authors and Affiliations
Contributions
Guanjie Jiang and Qin Zhang designed experiments and initiated research projects. Shizhang Zheng, Zhangjie Qin, Yupeng Yan, Hui Wang, and Qin Zhang provided supervision and writing-review and editing. Shuming Cao, Qiangjian Wu, and Lu Wang performed experiments and analyzed data. All authors contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible editor: Maria Manuela Abreu
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jiang, G., Cao, S., Zheng, S. et al. Effect of kaolinite on removal of Pb in simulated wastewater by oxalic acid–activated phosphate rock. J Soils Sediments 22, 1703–1712 (2022). https://doi.org/10.1007/s11368-022-03184-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-022-03184-1