Abstract
Purpose
Fire-induced changes in soil properties exert influence on soil processes, e.g., soil organic carbon (SOC) mineralization. The mineralization of organic substrates and soil priming effects in post-fire soils and the mechanisms involved remain elusive. This study aimed to investigate substrate mineralization with chemical recalcitrance gradient (sucrose, maize flour, and maize straw) and induced priming effects on forest soils after the fire.
Methods
Fire-burned forest soils (unburned as control) after 8 years were collected, and the physicochemical and biotic properties using high-throughput Illumina sequencing) were analyzed. Incubation of 42 days was conducted to investigate substrate decomposition and soil priming effects using the natural abundance 13C technique.
Results
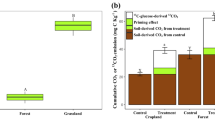
The bacterial community in soil after the fire event had high diversity and was dominated by the phyla of Actinobacteria, Proteobacteria, and Acidobacteria. The addition of substrate to the burned soil had larger mineralization and caused higher soil priming effects than the control soil. Positive priming of SOC by substrate was most likely attributed to “co-metabolism,” indicated by the positive correlation between soil priming and sucrose mineralization.
Conclusion
The intensity of substrate mineralization and soil priming effects in the burned soil depended on fire shifting microbial community and substrate quality itself.



Similar content being viewed by others
Change history
27 July 2021
A Correction to this paper has been published: https://doi.org/10.1007/s11368-021-03029-3
References
Aoyama M, Angers D, N’’dayegamiye A, Bissonnette N (2000) Metabolism of 13C-labeled glucose in aggregates from soils with manure application. Soil Biol Biochem 32:295–300
Bååth E, Frostegård Å, Pennanen T, Fritze H (1995) Microbial community structure and pH response in relation to soil organic matter quality in wood-ash fertilized, clear-cut or burned coniferous forest soils. Soil Biol Biochem 27:229–240
Bárcenas-Moreno G, García-Orenes F, Mataix-Solera J, Mataix-Beneyto J, Bååth E (2011) Soil microbial recolonisation after a fire in a Mediterranean forest. Biol Fert Soils 47:261–272
Bell JM, Smith JL, Bailey VL, Bolton H (2003) Priming effect and C storage in semi-arid no-till spring crop rotations. Biol Fert Soils 37:237–244
Bird MI, Wynn JG, Saiz G, Wurster CM, McBeath A (2015) The pyrogenic carbon cycle. Annual Rev Earth Planetary Sci 43:273–298
Blagodatskaya E, Blagodatsky SA, Anderson T-H, Kuzyakov Y (2007) Priming effects in chernozem induced by glucose and N in relation to microbial growth strategies. Applied Soil Ecol 37:95–105
Brewer CA, Chuang VJ, Masiello CA, Gonnermann H, Gao X, Dugan B, Driver LE, Panzacchi P, Zygourakis K, Davies CA (2014) New approaches to measuring biochar density and porosity. Biomass Bioenergy 66:176–185
Chen D, Wang Y, Lan Z, Li J, Xing W, Hu S, Bai Y (2015) Biotic community shifts explain the contrasting responses of microbial and root respiration to experimental soil acidification. Soil Biol Biochem 90:139–147
Chen L, Jiang Y, Liang C, Luo Y, Xu Q, Han C, Zhao Q, Sun B (2019) Competitive interaction with keystone taxa induced negative priming under biochar amendments. Microbiome 7:1–18
Cheng W (2009) Rhizosphere priming effect: its functional relationships with microbial turnover, evapotranspiration, and C-N budgets. Soil Biol Biochem 41:1795–1801
Cotrufo MF, Wallenstein MD, Boot CM, Denef K, Paul E (2013) The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: do labile plant inputs form stable soil organic matter? Global Change Biol 19:988–995
Coyne MS (1999) Soil microbiology: an exploratory approach. Delmar Publishers, Albany, NY, p 462
Docherty KM, Balser TC, Bohannan BJM, Gutknecht JLM (2012) Soil microbial responses to fire and interacting global change factors in a California annual grassland. Biogeochemistry 109(1–3):63–83
Fan F, Yin C, Tang Y, Li Z, Song A, Wakelin SA, Zou J, Liang Y (2014) Probing potential microbial coupling of carbon and nitrogen cycling during decomposition of maize residue by 13C-DNA-SIP. Soil Bioland Biochem 70:12–21
Fang Y, Singh B, Singh B, Krull E (2014) Biochar carbon stability in four contrasting soils. European J Soil Sci 65:60–71
Farrell M, Kuhn TK, Macdonald LM, Maddern TM, Murphy DV, Hall PA, Singh BP, Baumann K, Krull ES, Baldock JA (2013) Microbial utilisation of biochar-derived carbon. Sci Total Environ 465:288–297
Fontaine S, Barot S, Barré P, Bdioui N, Mary B, Rumpel C (2007) Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 450:277–280
Goldfarb KC, Karaoz U, Hanson CA, Santee CA, Bradford MA, Treseder KK, Wallenstein MD, Brodie EL (2011) Differential growth responses of soil bacterial taxa to carbon substrates of varying chemical recalcitrance. Front Microbiol 2(94):94
Graaff MAD, Classen AT, Castro HF, Schadt CW (2010) Labile soil carbon inputs mediate the soil microbial community composition and plant residue decomposition rates. New Phytol 188:1055–1064
Guenet B, Danger M, Abbadie L, Lacroix G (2010) Priming effect: bridging the gap between terrestrial and aquatic ecology. Ecol 91:2850–2861
Güsewell S, Gessner MO (2009) N : P ratios influence litter decomposition and colonization by fungi and bacteria in microcosms. Funct Ecol 23:211–219
Herath H, Camps-Arbestain M, Hedley M (2013) Effect of biochar on soil physical properties in two contrasting soils: an Alfisol and an Andisol. Geoderma 209:188–197
Hernandez-Soriano MC, Kerré B, Goos P, Hardy B, Dufey J, Smolders E (2016) Long-term effect of biochar on the stabilization of recent carbon: soils with historical inputs of charcoal. GCB Bioenergy 8:371–381
Hu L, Cao L, Zhang R (2014) Bacterial and fungal taxon changes in soil microbial community composition induced by short-term biochar amendment in red oxidized loam soil. World J Microbiol Biotechnol 30:1085–1092
Jagadamma S, Mayes MA, Steinweg JM, Schaeffer SM (2014) Substrate quality alters the microbial mineralization of added substrate and soil organic carbon. Biogeosciences 11:665–4678
Jien S-H, Wang C-S (2013) Effects of biochar on soil properties and erosion potential in a highly weathered soil. CATENA 110:225–233
Khodadad CL, Zimmerman AR, Green SJ, Uthandi S, Foster JS (2011) Taxa-specific changes in soil microbial community composition induced by pyrogenic carbon amendments. Soil Biol Biochem 43:385–392
Kim JS, Sparovek G, Longo RM, De Melo WJ, Crowley D (2007) Bacterial diversity of terra preta and pristine forest soil from the Western Amazon. Soil Biol Biochem 39:684–690
Kuzyakov Y, Schneckenberger K (2004) Review of estimation of plant rhizodeposition and their contribution to soil organic matter formation. Arch Agron Soil Sci 50:115–132
Kuzyakov Y, Subbotina I, Chen H, Bogomolova I, Xu X (2009) Black carbon decomposition and incorporation into soil microbial biomass estimated by 14C labeling. Soil Biol Biochem 41:210–219
Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D (2011) Biochar effects on soil biota–a review. Soil Biol Biochem 43:1812–1836
Lei Z, Li Q, Song X, Wang W, Zhang Z, Peng C, Tian L (2018) Biochar mitigates dissolved organic carbon loss but does not affect dissolved organic nitrogen leaching loss caused by nitrogen deposition in Moso bamboo plantations. Global Ecol Conserv 16:e00494
Liu Z, Dugan B, Masiello CA, Gonnermann HM (2017) Biochar particle size, shape, and porosity act together to influence soil water properties. PLOS One 12(6):e0179079
Luo X, Wang L, Liu G, Wang X, Wang Z, Zheng H (2016) Effects of biochar on carbon mineralization of coastal wetland soils in the Yellow River Delta, China. Ecol Eng 94:329–336
Luo Y, Durenkamp M, De Nobili M, Lin Q, Brookes P (2011) Short term soil priming effects and the mineralisation of biochar following its incorporation to soils of different pH. Soil Biol Biochem 43:2304–2314
Luo Y, Durenkamp M, De Nobili M, Lin Q, Devonshire B, Brookes P (2013) Microbial biomass growth, following incorporation of biochars produced at 350 C or 700 C, in a silty-clay loam soil of high and low pH. Soil Biol Biochem 57:513–523
Luo Y, Zang H, Yu Z, Chen Z, Gunina A, Kuzyakov Y, Xu J, Zhang K, Brookes PC (2017) Priming effects in biochar enriched soils using a three-source-partitioning approach: 14C labelling and 13C natural abundance. Soil Biol Biochem 106:28–35
Maestrini B, Nannipieri P, Abiven S (2015) A meta-analysis on pyrogenic organic matter induced priming effect. GCB Bioenergy 7:577–590
Mikutta R, Kleber M, Torn MS, Jahn R (2006) Stabilization of soil organic matter: association with minerals or chemical recalcitrance? Biogeochemistry 77:25–56
Mukherjee A, Zimmerman A, Hamdan R, Cooper W (2014) Physicochemical changes in pyrogenic organic matter (biochar) after 15 months of field aging. Solid Earth 5:693–704
Naisse C, Girardin C, Lefevre R, Pozzi A, Maas R, Stark A, Rumpel C (2015) Effect of physical weathering on the carbon sequestration potential of biochars and hydrochars in soil. GCB Bioenergy 7:488–496
Neff J, Reynolds R, Belnap J, Lamothe P (2005) Multi-decadal impacts of grazing on soil physical and biogeochemical properties in southeast Utah. Ecol Appl 15:87–95
Nottingham AT, Whitaker J, Turner BL, Salinas N, Zimmermann M, Malhi Y, Meir P (2015) Climate warming and soil carbon in tropical forests: insights from an elevation gradient in the Peruvian Andes. Bioscience 65:906–921
Noyce GL, Winsborough C, Fulthorpe R, Basiliko N (2016) The microbiomes and metagenomes of forest biochars. Rep 6(1):26425
O’’neill B, Grossman J, Tsai MT, Gomes JE, Lehmann J, Peterson J, Neves E, Thies JE (2009) Bacterial community composition in Brazilian Anthrosols and adjacent soils characterized using culturing and molecular identification. Microbial Ecol 58:23–35
Pausch J, Kuzyakov Y (2018) Carbon input by roots into the soil: quantification of rhizodeposition from root to ecosystem scale. Global Change Biol 24:1–12
Pauvert C, Buée M, Laval V, Edel-Hermann V, Fauchery L, Gautier A, Lesur I, Vallance J, Vacher C (2019) Bioinformatics matters: the accuracy of plant and soil fungal community data is highly dependent on the metabarcoding pipeline. Fungal Ecol 41:23–33
Pereira eSMC, Semenov AV, Schmitt H, van Elsas JD, Salles JF (2013) Microbe-mediated processes as indicators to establish the normal operating range of soil functioning. Soil Biol Biochem 57:995–1002
Pérez-Valera E, Verdú M, Navarro-Cano J, Goberna M (2020) Soil microbiome drives the recovery of ecosystem functions after fire. Soil Biol Biochem 149:107948
Pluchon N, Vincent AG, Gundale MJ, Nilsson MC, Kardol P, Wardle DA (2016) The impact of charcoal and soil mixtures on decomposition and soil microbial communities in boreal forest. Applied Soil Ecol 99:40–50
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO (2012) The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41:D590-D 596
Quin PR, Cowie AL, Flavel RJ, Keen BP, Macdonald LM, Morris SG, Singh BP, Young IM, Van Zwieten L (2014) Oil mallee biochar improves soil structural properties—a study with x-ray micro-CT. Agric Ecosyst Environ 191:142–149
Randerson JT, Chen Y, van der Werf GR, Rogers BM, Morton DC (2012) Global burned area and biomass burning emissions from small fires. J Geophys Res-Biogeo 117:G04012
Rousk J, Bååth E, Brookes PC, Lauber CL, Lozupone C, Caporaso JG, Knight R, Fierer N (2010) Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J 4:1340–1351
Rumpel C, Leifeld J, Santin C, Doerr S (2015) Movement of biochar in the environment. Biochar for environmental management. Sci Technol Implementat 283–300
Santín C, Doerr SH, Preston CM, González-Rodríguez G (2015) Pyrogenic organic matter production from wildfires: a missing sink in the global carbon cycle. Glob Chang Biol 21:1621–1633
Santos F, Torn MS, Bird JA (2012) Biological degradation of pyrogenic organic matter in temperate forest soils. Soil Biol Biochem 51:115–124
Singh BP, Cowie AL (2014) Long-term influence of biochar on native organic carbon mineralisation in a low-carbon clayey soil. Sci Rep 4:3687
Sun DL, Jiang X, Wu QL, Zhou NY (2013) Intragenomic heterogeneity of 16S rRNA genes causes overestimation of prokaryotic diversitY. Appl Environ Microb 79:5962–5969
Tamura M, Tharayil N (2014) Plant litter chemistry and microbial priming regulate the accrual, composition and stability of soil carbon in invaded ecosystems. New Phytol 203:110–124
Tarkalson DD, Kachman SD, Knops JMN, Thies JE, Wortmann CS (2008) Decomposition of Bt and non-Bt corn hybrid residues in the field. Nutr Cyc Agroecosys 80:211–222
Thies JE, Rillig MC (2009) Characteristics of biochar: biological properties. Biochar for Environmental Management: Sci Technol 1:85–105
Tinsley J, Taylor T, Moore J (1951) The determination of carbon dioxide derived from carbonates in agricultural and biological materials. Analyst 76:300–310
Van der Werf GR, Randerson JT, Giglio L, Collatz GJ, Mu M, Kasibhatla PS, DeFries MDC, RS. Jin Y, van Leeuwen TT (2010) Global fire emissions and the contribution of deforestation, savanna. forest, agricultural. and peat fires (1997–2009). Atmos Chem Phye 10:11707–11735
Ventura M, Alberti G, Viger M, Jenkins JR, Girardin C, Baronti S, Zaldei A, Taylor G, Rumpel C, Miglietta F (2015) Biochar mineralization and priming effect on SOM decomposition in two European short rotation coppices. GCB Bioenergy 7:1150–1160
Wang J, Xiong Z, Kuzyakov Y (2016) Biochar stability in soil: meta-analysis of decomposition and priming effects. GCB Bioenergy 8:512–523
Wang X, Butterly CR, Baldock JA, Tang C (2017) Long-term stabilization of crop residues and soil organic carbon affected by residue quality and initial soil pH. Sci the Total Environ 587:502–509
Wang XS (2010) Black carbon in urban topsoils of Xuzhou (China): environmental implication and magnetic proxy. Environ Monit Assess 163:41–47
Watzinger A, Feichtmair S, Kitzler B, Zehetner F, Kloss S, Wimmer B, Zechmeister-Boltenstern S, Soja G (2014) Soil microbial communities responded to biochar application in temperate soils and slowly metabolized 13C-labelled biochar as revealed by 13C PLFA analyses: results from a short-term incubation and pot experiment. Eur J Soil Sci 65:40–51
Weng ZH, Van Zwieten L, Singh BP, Tavakkoli E, Joseph S, Macdonald LM, Rose TJ, Rose MT, Kimber SW, Morris S (2017) Biochar built soil carbon over a decade by stabilizing rhizodeposits. Nat Clim Chang 7:371–376
Wetterstedt JÅM, Persson T, ÅGren GI, (2010) Temperature sensitivity and substrate quality in soil organic matter decomposition: results of an incubation study with three substrates. Global Change Biol 16:1806–1819
Whitman T, Pepe-Ranney C, Enders A, Koechli C, Campbell A, Buckley DH, Lehmann J (2016) Dynamics of microbial community composition and soil organic carbon mineralization in soil following addition of pyrogenic and fresh organic matter. ISME J 10:2918–2930
Wu JJRG, Joergensen RG, Pommerening B, Chaussod R, Brookes PC (1990) Measurement of soil microbial biomass C by fumigation-extraction-an automated procedure. Soil Biol Biochem 22(8):1167-1169
Yu Z, Chen L, Pan S, Li Y, Kuzyakov Y, Xu J, Brookes P, Luo Y (2018) Feedstock determines biochar-induced soil priming effects by stimulating the activity of specific microorganisms. Eur J Soil Sci 69:521–534
Zhang K, Chen L, Li Y, Brookes PC, Xu J, Luo Y (2020) Interactive effects of soil pH and substrate quality on microbial utilization. Eur J Soil Biol 96: 103151
Zheng H, Wang X, Luo X, Wang Z, Xing B (2018) Biochar-induced negative carbon mineralization priming effects in a coastal wetland soil: roles of soil aggregation and microbial modulation. Sci Total Environ 610:951–960
Zimmerman AR, Gao B, Ahn MY (2011) Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol Biochem 43:169–1179
Zimmerman AR, Ouyang L (2019) Priming of pyrogenic C (biochar) mineralization by dissolved organic matter and vice versa. Soil Biol Biochem 130:105–112
Funding
This study was supported by the National Natural Science Foundation of China (41761134095, 41671233).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Weixin Ding
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: In the original version of this article, the name of the 5th and 6th authors were incorrectly captured and should be changed to Peduruhewa H. Jeewani and Jianming Xu.
The original online version of this article was revised: In the original version of this article, the name of the 5th and 6th authors were incorrectly captured and should be changed to Peduruhewa H. Jeewani and Jianming Xu.
Supplementary Information
Below is the link to the electronic supplementary material.
11368_2021_3003_MOESM1_ESM.docx
Supplementary file1 Fig. S1 Cumulative Total CO2 (a), cumulative Substate derived CO2 (b) and Cumulative Primed soil CO2 (c) with the addition of three substrates during 42 days of incubation. B: burned soil, UB: unburned soil; S1: sucrose, S2: maize powder; S3: maize straw (DOCX 387 KB)
Rights and permissions
About this article
Cite this article
Zhang, J., Ling, L., Singh, B.P. et al. Decomposition of substrates with recalcitrance gradient, primed CO2, and its relations with soil microbial diversity in post-fire forest soils. J Soils Sediments 21, 3007–3017 (2021). https://doi.org/10.1007/s11368-021-03003-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-021-03003-z