Abstract
Purpose
Complete ammonia oxidation (comammox) bacteria can independently oxidize ammonia to nitrate, and their discovery has changed the long-term understanding of nitrification. Comammox bacteria have been found in a variety of natural environments, but there is still a lack of understanding regarding their presence in eutrophic lakes. The main purpose of this study was to investigate the diversity and abundance of comammox bacteria in a eutrophic lake and the effects of sewage discharge on bacterial abundance.
Materials and methods
Samples were taken from five areas of Lake Tangxun, China, i.e., a water chestnut (Trapa natans) area, a lotus (Nelumbo) area, a bare sediment area, a food sewage disposal area, and a domestic sewage disposal area. The diversity of comammox bacteria and the abundance of ammonia-oxidizing archaea (AOA), ammonia-oxidizing bacteria (AOB), comammox bacteria, and anaerobic ammonia-oxidizing (anammox) bacteria were measured.
Results and discussion
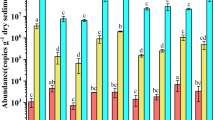
Comammox bacteria were widely found in Lake Tangxun and were closely related to Candidatus Nitrospira nitrosa and Candidatus Nitrospira inopinata. The abundance of the comammox amoA gene was lower in the two sewage discharge areas (1.3 × 107 copies g−1) than in the emergent plant areas (1.75 × 108 copies g−1) and the bare sediment area (1.0 × 108 copies g−1). The abundance of the comammox amoA gene at the five sampling areas was higher than that of the AOA amoA gene and AOB amoA gene, which indicated that comammox bacteria had a growth advantage in this eutrophic lake.
Conclusions
Comammox bacteria were found in eutrophic lake sediments, and these bacteria belonged to comammox clade A. The abundance of comammox bacteria in the two sewage discharge areas was the lowest, indicating that they could survive in eutrophic waters, but eutrophication inhibited their growth.



Similar content being viewed by others
References
Camejo PY, Domingo JS, McMahon KD, Noguera DR (2017) Genome-enabled insights into the ecophysiology of the comammox bacterium Candidatus Nitrospira nitrosa. mSystems 2:16
Chen YL, Xu ZW, Hu HW, Hu YJ, Hao ZP, Jiang Y, Chen BD (2013) Responses of ammonia-oxidizing bacteria and archaea to nitrogen fertilization and precipitation increment in a typical temperate steppe in Inner Mongolia. Appl Soil Ecol 68:36–45
Christensen PB, Revsbech NP, Sandjensen K (1994) Microsensor analysis of oxygen in the rhizosphere of the aquatic macrophyte Littorella-Uniflora (L) ascherson. Plant Physiol 105:847–852
Costa E, Pérez J, Kreft J-U (2006) Why is metabolic labour divided in nitrification? Trends Microbiol 14:213–219
Daims H, Lebedeva EV, Pjevac P, Han P, Herbold C, Albertsen M, Jehmlich N, Palatinszky M, Vierheilig J, Bulaev A, Kirkegaard RH, von Bergen M, Rattei T, Bendinger B, Nielsen PH, Wagner M (2015) Complete nitrification by Nitrospira bacteria. Nature 528:504–509
Di HJ, Cameron KC, Shen JP, Winefield CS, O'Callaghan M, Bowatte S, He JZ (2009) Nitrification driven by bacteria and not archaea in nitrogen-rich grassland soils. Nat Geosci 2:621–624
Di HJ, Cameron KC, Shen JP, Winefield CS, O'Callaghan M, Bowatte S, He JZ (2010) Ammonia-oxidizing bacteria and archaea grow under contrasting soil nitrogen conditions. FEMS Microbiol Ecol 72:386–394
Duan HR, Wang QL, Erler DV, Ye L, Yuan ZG (2018) Effects of free nitrous acid treatment conditions on the nitrite pathway performance in mainstream wastewater treatment. Sci Total Environ 644:360–370
Fowler SJ, Palomo A, Dechesne A, Mines PD, Smets BF (2018) Comammox Nitrospira are abundant ammonia oxidizers in diverse groundwater-fed rapid sand filter communities. Environ Microbiol 20:1002–1015
Gao JF, Fan XY, Pan KL, Li HY, Sun LX (2016) Diversity, abundance and activity of ammonia-oxidizing microorganisms in fine particulate matter. Sci Rep 6:38785
Haichar FE, Santaella C, Heulin T, Achouak W (2014) Root exudates mediated interactions belowground. Soil Biol Biochem 77:69–80
Herrmann M, Saunders AM, Schramm A (2009) Effect of lake trophic status and rooted macrophytes on community composition and abundance of ammonia-oxidizing prokaryotes in freshwater sediments. Appl Environ Microb 75:3127–3136
Hou J, Song CL, Cao XY, Zhou YY (2013) Shifts between ammonia-oxidizing bacteria and archaea in relation to nitrification potential across trophic gradients in two large Chinese lakes (Lake Taihu and Lake Chaohu). Water Res 47:2285–2296
Ji GD, Wang RJ, Zhi W, Liu XX, Kong YP, Tan YF (2012) Distribution patterns of denitrification functional genes and microbial floras in multimedia constructed wetlands. Ecol Eng 44:179–188
Jiang Q, Xia F, Zhu T, Wang D, Quan Z (2019) Distribution of comammox and canonical ammonia oxidizing bacteria in tidal flat sediments of the Yangtze River estuary at different depths over four seasons. J Appl Microbiol 127:533–543
Keene-Beach N, Noguera DR (2019) Design and assessment of species-level qPCR primers targeting comammox. Front Microbiol 10:36
Kits KD, Sedlacek CJ, Lebedeva EV, Han P, Bulaev A, Pjevac P, Daebeler A, Romano S, Albertsen M, Stein LY, Daims H, Wagner M (2017) Kinetic analysis of a complete nitrifier reveals an oligotrophic lifestyle. Nature 549:269–272
Koch H, Kessel MAHJV, Sebastian L (2019) Complete nitrification: insights into the ecophysiology of comammox nitrospira. Appl Microbiol Biot 103:177–189
Konneke M, Bernhard AE, de la Torre JR, Walker CB, Waterbury JB, Stahl DA (2005) Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 437:543–546
Kuypers MMM, Lavik G, Woebken D, Schmid M, Fuchs BM, Amann R, Jorgensen BB, Jetten MSM (2005) Massive nitrogen loss from the Benguela upwelling system through anaerobic ammonium oxidation. P Natl Acad Sci USA 102:6478–6483
Li P, Li SN, Zhang Y, Cheng HM, Zhou HL, Qiu LG, Diao XP (2018) Seasonal variation of anaerobic ammonium oxidizing bacterial community and abundance in tropical mangrove wetland sediments with depth. Appl Soil Ecol 130:149–158
Liu B, Li YM, Zhang JP, Zhou XH, Wu CD (2014) Abundance and diversity of ammonia-oxidizing microorganisms in the sediments of Jinshan Lake. Curr Microbiol 69:751–757
Meinhardt KA, Bertagnolli A, Pannu MW, Strand SE, Brown SL, Stahl DA (2015) Evaluation of revised polymerase chain reaction primers for more inclusive quantification of ammonia-oxidizing archaea and bacteria. Env Microbiol Rep 7:354–363
Nikolausz M, Kappelmeyer U, Szekely A, Rusznyak A, Marialigeti K, Kastner M (2008) Diurnal redox fluctuation and microbial activity in the rhizosphere of wetland plants. Eur J Soil Biol 44:324–333
Orellana LH, Chee-Sanford JC, Sanford RA, Loffler FE, Konstantinidis KT (2018) Year-round shotgun metagenomes reveal stable microbial communities in agricultural soils and novel ammonia oxidizers responding to fertilization. Appl Environ Microb 84:e01646–e01617
Oshiki M, Satoh H, Okabe S (2016) Ecology and physiology of anaerobic ammonium oxidizing bacteria. Environ Microbiol 18:2784–2796
Otte S, Schalk J, Kuenen JG, Jetten MSM (1999) Hydroxylamine oxidation and subsequent nitrous oxide production by the heterotrophic ammonia oxidizer Alcaligenes faecalis. Appl Microbiol Biot 51:255–261
Palomo A, Pedersen AG, Fowler SJ, Dechesne A, Sicheritz-Ponten T, Smets BF (2018) Comparative genomics sheds light on niche differentiation and the evolutionary history of comammox Nitrospira. ISME J 12:1779–1793
Pan KL, Gao JF, Fan XY, Li DC, Dai HH (2018) The more important role of archaea than bacteria in nitrification of wastewater treatment plants in cold season despite their numerical relationships. Water Res 145:552–561
Pinto AJ, Marcus DN, Ijaz UZ, Santos QMBD, Dick GJ, Raskin L (2016) Metagenomic evidence for the presence of comammox Nitrospira-like bacteria in a drinking water system. Msphere 1:e00054–e00015
Pjevac P, Schauberger C, Poghosyan L, Herbold CW, van Kessel MAHJ, Daebeler A, Steinberger M, Jetten MSM, Lucker S, Wagner M, Daims H (2017) AmoA-targeted polymerase chain reaction primers for the specific detection and quantification of comammox Nitrospira in the environment. Front Microbiol 8:1508
Roots P, Wang YB, Rosenthal AF, Griffin JS, Sabba F, Petrovich M, Yang FH, Kozak JA, Zhang H, Wells GF (2019) Comammox Nitrospira are the dominant ammonia oxidizers in a mainstream low dissolved oxygen nitrification reactor. Water Res 157:396–405
Shi XZ, Hu HW, Wang JQ, He JZ, Zheng CY, Wan XH, Huang ZQ (2018) Niche separation of comammox Nitrospira and canonical ammonia oxidizers in an acidic subtropical forest soil under long-term nitrogen deposition. Soil Biol Biochem 126:114–122
Tsiknia M, Tzanakakis VA, Paranychianakis NV (2013) Insights on the role of vegetation on nitrogen cycling in effluent irrigated lands. Appl Soil Ecol 64:104–111
van Kessel MAHJ, Speth DR, Albertsen M, Nielsen PH, Op den Camp HJM, Kartal B, Jetten MSM, Lucker S (2015) Complete nitrification by a single microorganism. Nature 528:555–559
Wang YF, Gu JD (2013) Higher diversity of ammonia/ammonium-oxidizing prokaryotes in constructed freshwater wetland than natural coastal marine wetland. Appl Microbiol Biot 97:7015–7033
Wang YL, Ma LP, Mao YP, Jiang XT, Xia Y, Yu K, Li B, Zhang T (2017) Comammox in drinking water systems. Water Res 116:332–341
Wang MY, Huang GH, Zhao ZR, Dang CY, Liu W, Zheng MS (2018) Newly designed primer pair revealed dominant and diverse comammox amoA gene in full-scale wastewater treatment plants. Bioresour Technol 270:580–587
Xia F, Wang JG, Zhu T, Zou B, Rhee SK, Quan ZX (2018) Ubiquity and diversity of complete ammonia oxidizers (Comammox). Appl Environ Microb 84:e01390–e01318
Yan J, Haaijer SCM, den Camp HJMO, van Niftrik L, Stahl DA, Konneke M, Rush D, Damste JSS, Hu YY, Jetten MSM (2012) Mimicking the oxygen minimum zones: stimulating interaction of aerobic archaeal and anaerobic bacterial ammonia oxidizers in a laboratory-scale model system. Environ Microbiol 14:3146–3158
Yu CD, Hou LJ, Zheng YL, Liu M, Yin GY, Gao J, Liu C, Chang YK, Han P (2018) Evidence for complete nitrification in enrichment culture of tidal sediments and diversity analysis of clade a comammox Nitrospira in natural environments. Appl Microbiol Biot 102:9363–9377
Zhang JP, Liu B, Zhou XH, Chu JY, Li YM, Wang MY (2015) Effects of emergent aquatic plants on abundance and community structure of ammonia-oxidising microorganisms. Ecol Eng 81:504–513
Zhao DY, Luo J, Zeng J, Wang M, Yan WM, Huang R, Wu QL (2014) Effects of submerged macrophytes on the abundance and community composition of ammonia-oxidizing prokaryotes in a eutrophic lake. Environ Sci Pollut Res Int 21:389–398
Zhao ZR, Huang GH, He SS, Zhou N, Wang MY, Dang CY, Wang JW, Zheng MS (2019) Abundance and community composition of comammox bacteria in different ecosystems by a universal primer set. Sci Total Environ 691:146–155
Zheng MS, Wang MY, Zhao ZR, Zhou N, He SS, Liu SF, Wang JW, Wang XK (2019) Transcriptional activity and diversity of comammox bacteria as a previously overlooked ammonia oxidizing prokaryote in full-scale wastewater treatment plants. Sci Total Environ 656:717–722
Funding
This work was supported by the National Natural Science Foundation of China (41371452, 40901264).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that no conflict of interest exists in the submission of this article and the article does not contain any studies with human participants or animals. The manuscript is approved by all authors for publication.
Additional information
Responsible editor: Terrence H. Bell
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 143 kb)
Rights and permissions
About this article
Cite this article
Xu, Y., Liu, G., Hua, Y. et al. The diversity of comammox bacteria and the effect of sewage discharge on their abundance in eutrophic lake sediments. J Soils Sediments 20, 2495–2503 (2020). https://doi.org/10.1007/s11368-020-02618-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-020-02618-y