Abstract
Purpose
Tetracycline (TC) is one of the most used antibiotics and has accumulated in soil. Its adsorption behavior was studied, and the effect of dissolved organic matter (DOM) was evaluated based on the molecular interaction between DOM and TC. This paper is helpful for further understanding the mobility and bioavailability of TC in soil.
Materials and methods
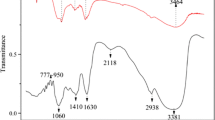
Physiochemical properties of the soil samples (named as QM-SA soil) were measured. DOM was extracted from QM-SA soil. The adsorption of TC on QM-SA soil and QM-SA soil after DOM extracted was studied based on the batch experiments. Three-dimensional excitation–emission matrix (3D-EEM), synchronous fluorescence, two-dimensional correlation spectroscopy (2D-COS) and FT-IR (Fourier transform infrared spectroscopy) were used to reveal the binding of DOM with TC.
Results and discussion
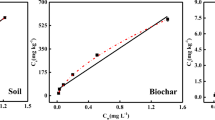
The maximum adsorption amount (qm) and the initial adsorption rate (K2q2e,cal) were calculated to be 5.241 mg/g and 2.491 mg/(h·g) by the Langmuir model and the pseudo-second-order kinetic model, respectively. Once DOM was extracted from QM-SA soil, the values of qm and K2q2e,cal for QM-SA soil after DOM extracted reduced to 1.274 mg/g and 1.257 mg/(h·g), decreased by 76% and 49% compared with 5.241 mg/g and 2.491 mg/(h·g), respectively. 3D-EEM demonstrated that DOM contained humic-like, protein-like, soluble microbial by-product-like and fulvic-like substances, which bound with TC and contributed to the formation of substance-TC-complexes. In order to find out which substance in DOM has the strongest affinity for tetracycline, synchronous fluorescence, 2D-COS and the site-binding model were used. Protein-like substance bound with TC more strongly than humic-like substance. As for protein-like substances, tryptophan-like substance exhibited higher affinity to TC than tyrosine-like substance. The order of the binding affinity was tryptophan > tyrosine > humic–like substance. FTIR proved that, besides these fluorescent substances, polysaccharide-like substance and aliphatic compound without fluorescence were also responsible for binding with TC.
Conclusions
When the initial concentration of TC ranged from 5 to 40 mg/L, all the removal efficiencies were more than 60% at the equilibrium time. The retention degree of TC in QM-SA soil was strong. The ratio of qm between QM-SA soil after DOM extracted and QM-SA soil was about 25%, that is, the effect of DOM on the adsorption of TC was significant.






Similar content being viewed by others
References
Bai L, Zhao Z, Wang CL, Wang CH, Liu X, Jiang HL (2017) Multi-spectroscopic investigation on the complexation of tetracycline with dissolved organic matter derived from algae and macrophyte. Chemosphere 187:421–429
Baker A (2002) Fluorescence properties of some farm wastes: implications for water quality monitoring. Water Res 36:0–195
Bansal OP (2012) A laboratory study on degradation studies of tetracycline and chlortetracycline in soils of Aligarh district as influenced by temperature, water content, concentration of farm yield manure, nitrogen and tetracyclines. P Natl A Sci India 82:503–509
Chao Y (2014) Adsorption of OTC on soils. Doctoral dissertation
Chen W, Westerhoff P, Leenheer JA, Booksh K (2003) Fluorescence excitation-emission matrix regional integration to quantify spectra for dissolved organic matter. Environ Sci Technol 37:5701–5710
Cheng G, Karthikeyan KG (2008) Sorption of the antibiotic tetracycline to humic-mineral complexes. J Environ Qual 37:704–711
Chopra I (2001) Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev 65:232–260
Dubin NH, Parmley TH, Ghodgaonkar RB, King TM (1984) Comparative effects of intrauterine instillation of analogues of quinacrine and tetracycline on uterine morphology in the rat. Contraception 29:553–559
ESPAUR (English surveillance programme for antimicrobial utilisation and resistance), (2018). https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/759975/ESPAUR_2018_report.pdf
Fan YR, Zheng CL, Hu AD, Wang QR, Shen ZX, Xue ZW, He C (2019) Investigating the binding properties between antimony(V) and dissolved organic matter (DOM) under different pH conditions during the soil sorption process using fluorescence and FTIR spectroscopy. Ecotox Environ Safe 181:34–42
Haverkort AJ, Mulder A, Waart MVD (1993) The effect of soil pH on yield losses caused by the potato cyst nematode globodera pallida. Potato Res 36:219–226
Hillis DG, Fletcher J, Solomon KR, Sibley PK (2011) Effects of ten antibiotics on seed germination and root elongation in three plant species. Arch Environ Cont Tox 60:220–232
Ho YS (2006) Review of second-order models for adsorption systems. Cheminform 136:681–689
Hu X, Zhou Q, Luo Y (2010) Occurrence and source analysis of typical veterinary antibiotics in manure, soil, vegetables and groundwater from organic vegetable bases, northern China. Environ Pollut 158:2992–2998
Huang SL, He S, Wei X, Xue G, Gao P (2015) Pollution characteristics of tetracycline residues and tetracycline resistance genes in sewage treatment plants:a review. Chem Ind Eng Prog 43:232–239
Hur J, Lee BM (2011) Characterization of binding site heterogeneity for copper within dissolved organic matter fractions using two-dimensional correlation fluorescence spectroscopy. Chemosphere 83:1603–1611
Hu YJ, Li Y, Zhang LX, Zhao RM, Qu SS (2005) Studies on the interaction between colchicine and bovine serum albumin by fluorescence quenching method. J Mol Struct 750:174–178
Ila'Ava VP, Asher CJ, Blamey FPC (1999) Response of sweet potato cultivars to acid soil infertility factors. I. Effects of solution pH on early growth. Crop Pasture Sci 51:23–28
Kalbitz K, Solinger S, Park JH, Michalzik B, Matzner E (2000) Controls on the dynamics of dissolved organic matter in soils: a review. Soil Sci 165:277–304
Kavita K, Mishra A, Jha B (2013) Extracellular polymeric substances from two biofilm forming Vibrio species: characterization and applications. Carbohyd Polym 94(2):882–888
Liu X, Lv H, Xu HC (2015) Two-dimension fluorescence correlation spectroscopy to characterize the binding of organic ligands with zinc in eutrophic lake. Chinese Chem Lett 197:205–209
Liu PP, Wang QR, Zheng CL, He C (2017) Sorption of sulfadiazine, norfloxacin, metronidazole, and tetracycline by granular activated carbon: kinetics, mechanisms, and isotherms. Water Air Soil Poll 228:129
Lin H, Sun W, Zhang Z, Chapman SJ, Freitag TE, Fu J, Zhang X, Ma JW (2016) Effects of manure and mineral fertilization strategies on soil antibiotic resistance gene levels and microbial community in a paddy-upland rotation system. Environ Pollut 211:332–337
Noda I, Liu Y, Ozaki Y, Czarnecki MA (1995) Two-dimensional Fourier transform near-infrared correlation spectroscopy studies of temperature-dependent spectral variations of Oleyl alcohol. J Phys Chem 99:3068–3073
Qiao M, Chen W, Su J, Zhang B, Zhang C (2012) Fate of tetracyclines in swine manure of three selected swine farms in China. J Environ Sci-China 24:1047–1052
Russell J (1961) Soil fertility changes in the long-term experimental plots at Kybybolite, South Australia. III. Changes in cation exchange capacity and exchangeable cations. Aust J Agric Res 12:273–285
Sakala GM, Rowell DL, Pilbeam CJ (2004) Acid–base reactions between an acidic soil and plant residues. Geoderma 123:0–232
Sarmah AK, Meyer MT, Boxall AA (2006) Global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 65:725–759
Sassman SA, Lee LS (2005) Sorption of three tetracyclines by several soils:assessing the role of ph and cation exchange. Environ Sci Technol 39:7452–7459
Song WJ, Mu GJ, Zhang DY, Pan XL (2010) Interaction of acetamiprid with extracellular polymeric substances (eps) from activated sludge:a fluorescence study. Afr J Biotechnol 9:7667–7673
Thiele-Bruhn S (2010) Pharmaceutical antibiotic compounds in soils-a review. J Plant Nutr Soil Sc 166:145–167
Thiele-Bruhn S, Beck IC (2005) Effects of sulfonamide and tetracycline antibiotics on soil microbial activity and microbial biomass. Chemosphere 59:0–465
Verlag S (2006) Principles of fluorescence spectroscopy. Die Naturwissenschaften 78:456
Wan Y, Bao Y, Zhou Q (2010) Simultaneous adsorption and desorption of cadmium and tetracycline on cinnamon soil. Chemosphere 80:807–812
Wang ML, Li J, Zhu ZZ, Guo XX (2010a) Advances in research on dissolved organic matter in soils. Bu lletin of mineralogy. Petrol Geochem 29(3):304–316
Wang QR, Zheng CC, Shen ZX, Lu Q, He C, Zhang TC, Liu JH (2019) Polyethyleneimine and carbon disulfide co-modified alkaline lignin for removal of Pb2+ ions from water. Chem Eng J 359:265–274
Wang Y, Zhang X, Zhang X, Meng Q, Gao F, Zhang Y (2017) Characterization of spectral responses of dissolved organic matter (DOM) for atrazine binding during the sorption process onto black soil. Chemosphere 180:531–539
Wu T, Zhou M, Guo H, Duan H, Chen H (2008) Adsorption of tetracycline on loess soils. Acta Sci Circumst 28:2311–2314
Yan L, Pan DY, Jiang XX, Ji XN, Yang HH, Li SQ (2017) Adsorption behaviour of tetracycline antibiotics in black soil and albic soil. J Northeast Agric Univ 48:57–62
Zhan XX, Zhou LX, Yang H, Jiang TH (2007) Infrared spectroscopy of DOM-PAHs complexes. Acta Pedol Sin 44(1):47–53
Wang JW, Sun RJ, Xiao AY, Wang SQ, Liu X, Zhou DM (2010b) Phosphate affects the adsorption of tetracycline on two soils with different characteristics. Geoderma 156(3–4):237–242
Zhao YP, Tan YY, Guo Y, Gu XY, Wang XR, Zhang Y (2013) Interactions of tetracycline with Cd (ii), Cu (ii) and Pb (ii) and their cosorption behavior in soils. Environ Pollut 180:206–213
Zhang QQ, Ying GG, Pan CG, Liu YS, Zhao JL (2015) Comprehensive evaluation of antibiotics emission and fate in the river basins of China: source analysis, multimedia modeling, and linkage to bacterial resistance. Environ Sci Technol 49:6772–6782
Zhang Y, Wang YF, Zhang XY, Li RZ, Chen YK, Meng QJ (2017) Investigating the behavior of binding properties between dissolved organic matter (DOM) and Pb2+ during the soil sorption process using parallel factor analysis (PARAFAC) andtwo-dimensional correlation spectroscopy (2D-COS). Environ Sci Pollut Res 24:25156–25165
Zhang T (2015) Adsọrption/desorption behavior of tetraçycline antibioticsin soịl. Doctoral dissertation
Zhang H, Li L, Zhou S (2014) Kinetic modeling of antimony(v) adsorption-desorption and transport in soils. Chemosphere 111:434–440
Zeng QY, Ding D, Tan X (2018) Pollution status and sources of tetracycline antibiotics in agricultural soil in China: a review. Ecol Environ Sci 27:194–202
Acknowledgments
The author would like to thank the Instrument Analysis Center of Xi’an Jiaotong University and Shaanxi Provincial Land Engineering Construction Group Co., Ltd. for technical support as well as the editor and anonymous reviewers for their valuable comments.
Funding
This work was financially supported by the National Natural Science Foundation of China (NO. 31871889), China Postdoctoral Science Foundation (2016 M602830), Shaanxi Postdoctoral Science Foundation (2016BSHTDZZ02), and Fundamental Research Fund for the Central Universities (xjj2016046).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Xilong Wang
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cheng, X., Hou, H., Li, R. et al. Adsorption behavior of tetracycline on the soil and molecular insight into the effect of dissolved organic matter on the adsorption. J Soils Sediments 20, 1846–1857 (2020). https://doi.org/10.1007/s11368-019-02553-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-019-02553-7