Abstract
Purpose
This study utilizes column tests to investigate the retardation of certain herbicides with different hydrophobicities (atrazine, alachlor and trifluralin) during transport through surface Danube sediment. The influence of water matrix on the retardation factor Rd and Freundlich constant Kf is investigated. The results are compared with batch tests to establish whether different methodologies result in similar or different conclusions.
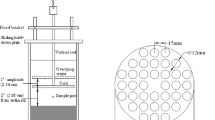
Materials and methods
A stainless steel column was filled with natural Danube sediment. Three water matrices were investigated: synthetic, Danube surface, and deep groundwater rich in natural organic matter (NOM). The goal was to examine whether different water matrices would result in changes in the Rd and corresponding Kf values. After a tracer experiment, single herbicide solutions were tested in the three water matrices. Herbicides were analyzed by gas chromatography coupled with electron capture detector (GC/ECD). Retardation factors obtained in the column experiments were calculated using Transmod software (version 2.2). The Kf values calculated were compared with the values obtained in previous batch experiments.
Results and discussion
A breakthrough curve (BTC) for trifluralin could not be obtained during the experiment. Atrazine Rd values were almost the same in the natural matrices (54 and 55 for the ground and surface waters, respectively), and lower in the synthetic water (40). Alachlor Rd values in the three water matrices were very similar (30–35). The corresponding Kf values for alachlor (8.47–17.4) were lower than those of atrazine (13.5–27.9). These results differ from those obtained by earlier batch tests, which showed similar Kf values for both atrazine (4.4–9.2) and alachlor (4.43–10.35) in all three matrices. In contrast to the results observed during the batch tests, the column tests exhibited higher Kf values in the natural water matrices than the synthetic water, possibly due to the influence of dissolved organic carbon on herbicide sorption.
Conclusions
Of the three herbicides investigated, the smallest retardation was observed for alachlor. This was unexpected given the relative hydrophobicities of alachlor and atrazine. The potential risk of transport through the sediment may therefore be greater for alachlor than the other two herbicides. This was indicated neither by the batch tests nor from the Koc–Kow estimations. Both herbicides exhibited similar Kd and Kf values in the batch tests, and lower values in the natural water matrices. In comparison, the column tests showed higher Kf values, with higher values in the natural matrices than in the synthetic water matrix.


Similar content being viewed by others
References
Amiri F, Börnick H, Worch E (2005) Sorption of phenols onto sandy aquifer material: the effect of dissolved organic matter (DOM). Water Res 39:933–941
Baker JR, Mihelcic JR, Luehrs DC, Hickey JP (1997) Evaluation of estimation methods for organic carbon normalized sorption coefficients. Water Environ Res 69:136–145
Banzhaf S, Hebig KH (2016) Use of column experiments to investigate the fate of organic micropollutants—a review. Hydrol Earth Syst Sci 20:3719–3737
Bi E, Zhang L, Schmidt TC, Haderlein SB (2009) Simulation of nonlinear sorption of N-heterocyclic organic contaminates in soil columns. J Contam Hydrol 107:58–65
Bi E, Schmidt TC, Haderlein SB (2010) Practical issues relating to soil column chromatography for sorption parameter determination. Chemosphere 80:787–793
Chefetz B, Bilkis Y, Polubesova T (2004) Sorption-desorption behavior of triazine and phenylurea herbicides in Kishon river sediments. Water Res 38:4383–4394
EC (2000) Council Directive 2000/60/EC (2000) OJ L327/1, 22.12.2000, European Commission, Luxembourg, pp 1–72
EC (2011) Guidance Document No. 27, Technical Guidance for Deriving Environmental Quality Standards, Technical Report—2011-055, European Commission, Luxembourg
EU (2013) Council Directive 213/39/EU (2013) OJ L 226/1, 12.8.2013, European Commision, Luxembourg, pp 1–17
Fernandez-Ramos C, Rodriguez-Gomez R, Reis MS, Zafra-Gomez A, Verge C, de Ferrer JA, Perez-Pascual M, Vilchez JL (2017) Sorption, degradation and transport phenomena of alcohol ethoxysulfates in agricultural soils. Laboratory studies. Chemosphere 171:661–670
Gerstl Z (1990) Estimation of organic chemical sorption by soils. J Contam Hydrol 6:357–375
Grünheid S, Amy G, Jekel M (2005) Removal of bulk dissolved organic carbon (DOC) and trace organic compounds by bank filtration and artificial recharge. Water Res 39:3219–3228
Hassett JJ, Banwart WL, Griffen RA (1983) Correlation of compound properties with sorption characteristics of nonpolar compounds by soil and sediments: concept and limitations. In: Francis CW, Auerbach SI (eds) Environmental and solid wastes: characterization, treatment and disposal. Butterworth Publishers, Newton, pp 161–178
Hur J, Lee B, Shin H (2011) Microbial degradation of dissolved organic matter (DOM) and its influence on phenanthrene–DOM interactions. Chemosphere 85:1360–1367
Jekel M, Gruenheid S (2006) Bank filration and groundwater recharge for treatment of polluted surface waters. In: Gimbel R, Graham NJD, Collins MR (eds) Recent progress in slow sand and alternative biofiltration processess. IWA Publishing, pp 519–529
Ju D, Young TM (2005) The influence of natural organic matter rigidity on the sorption, desorption, and competitive displacement rates of 1,2-dichlorobenzene. Environ Sci Technol 39:7956–7963
Karickhoff SW, Brown DS, Scott TA (1979) Sorption of hydrophobic pollutants on natural sediments. Water Res 13:241–248
Kenaga EE, Goring CAI (1980) Relationship between water solubility, soil sorption, octanol–water partitioning, and concentration of chemicals in biota. In: Eaton AC, Parrish JG, Hendricks PR (eds) Aquatic Toxicology ASTM STP 707. American Society for Testing and Materials, Philadelphia, p 405
Kragulj M, Dalmacija B, Tričković J, Dalmacija M, Maletić S, Krčmar D, Molnar J, Kerkez Đ, Pešić V (2011) Monitoring of priority substances in the water and sediment of protected zones and surface waters in AP Vojvodina. 40th National Conference with international participation „Voda 2011″, Zlatibor, Serbia, pp 109–114
Kragulj M, Tričković J, Dalmacija B, Ivančev-Tumbas I, Leovac A, Molnar J, Krčmar D (2014) Sorption of benzothiazoles onto sandy aquifer material under equilibrium and nonequilibrium conditions. J Serb Chem Soc 79:89–100
Kret E, Kiecak A, Malina G, Nijenhuis I, Postawa A (2015) Identification of TCE and PCE sorption and biodegradation parameters in a sandy aquifer for fate and transport modeling: batch and column studies. Environ Sci Pollut Res 22:9877–9888
Leovac A, Vasyukova E, Ivancev-Tumbas I, Uhl W, Kragulj M, Tričković J, Kerkez Đ, Dalmacija B (2015) Sorption of atrazine, alachlor and trifluralin from water onto different geosorbents. RSC Adv 25:8122–8133
Löfler D, Römbke J, Meller M, Ternes T (2005) Environmental fate of pharmaceuticals in water/sediment systems. Environ Sci Technol 39:5209–5218
Maeng SK, Sharma SK, Lekkerkerker-Teunissen K, Amy GL (2011) Occurence and fate of bulk organic matter and pharmaceuticaly active compounds in managed aquifer recharge: a review. Water Res 45:3015–3033
Murillo-Torres R, Duran-Alvarez JC, Prado B, Jimenez-Cisneros BE (2012) Sorption and mobility of two micropollutants in three agricultural soils: a comparative analysis of their behavior in batch and column experiments. Geoderma 189-190:462–468
Radovic T, Grujic S, Petkovic A, Dimkic M, Lausevic M (2015) Determination of pharmaceuticals and pesticides in river sediments and corresponding surface and ground water in the Danube River and tributaries in Serbia. Environ Monit Assess 187:4092
Rahman M, Worch E (2005) Nonequilibrium sorption of phenols onto geosorbents: the impact of pH on intraparticle mass transfer. Chemosphere 61:1419–1426
Rahman M, Amiri F, Worch E (2003) Application of the mass transfer model for describing nonequilibrium transport of HOCs through natural geosorbents. Water Res 37:4673–4684
Schmidt CK, Lange FT, Brauch HJ (2006) Assessing the impact of local boundary conditions on the fate of organic micropollutants during undergo passage. In: Gimbel R, Graham NJD, Collins MR (eds) Recent progress in slow sand and alternative biofiltration processess. IWA Publishing, London, pp 561–569
Schwarzenbach RP, Westall J (1981) Transport of nonpolar organic compounds from surface water to groundwater. Laboratory sorption studies. Environ Sci Technol 15:1360–1367
Schwarzenbach RP, Escher BI, Fenner K, Hofstetter TB, Johnson CA, von Gunten U, Wehrli B (2006) The challenge of micropollutants in aquatic systems. Science 313(5790):1072–1077
SEPA (2012) Results of testing the quality of surface and groundwater for 2011. Serbian Environmental Protection Agency, Ministry of Environmental Protection, Serbia
SEPA (2013) Results of testing the quality of surface and groundwater for 2012. Serbian Environmental Protection Agency, Ministry of Environmental Protection, Serbia
SEPA (2014) Results of testing the quality of surface and groundwater for 2013. Serbian Environmental Protection Agency, Ministry of Environmental Protection, Serbia
SEPA (2015) Results of testing the quality of surface and groundwater for 2014. Serbian Environmental Protection Agency, Ministry of Environmental Protection, Serbia
SEPA (2017a) Results of testing the quality of surface and groundwater for 2015. Serbian Environmental Protection Agency, Ministry of Environmental Protection, Serbia
SEPA (2017b) Results of testing the quality of surface and groundwater for 2016. Serbian Environmental Protection Agency, Ministry of Environmental Protection, Serbia
Sun K, Gao B, Zhang Z, Zhang G, Zhao Y, Xing B (2010) Sorption of atrazine and phenanthrene by organic matter fractions in soil and sediment. Environ Pollut 158:3520–3526
Talete srl DRAGON (2011) (Software for Molecular Descriptor Calculation) Version 6.0, 2011 (http://talete.mi.it)
Tan G, Sun W, Xu Y, Wang H, Xu N (2016) Sorption of mercury (II) and atrazine by biochar, modified biochars and biochar based activated carbon in aqueous solution. Bioresour Technol 211:727–735
Toxicology Data Network (2003) Hazardous Substances Databank Number: 1003. U.S. National Library of Medicine, 8600 Rockville Pike, Bethesda, MD, USA. http://toxnet.nlm.nih.gov/. Assessed 15 March 2018
Tričković J, Kragulj Isakovski M, Watson M, Maletić S, Rončević S, Dalmacija B, Konya Z, Kukovecz A (2016) Sorption behaviour of trichlorobenzenes and polycyclic aromatic hydrocarbons in the absence or presence of carbon nanotubes in the aquatic environment. Water Air Soil Pollut 227:374
Worch E (2004) Modeling of the solute transport under nonequilibrium conditions on the basis of mass transfer equations. J Contam Hydrol 68:97–120
Worch E (2006) Program TransMod (Version 2.2) Program Documentation
Worch E (2012) Adsorption technology in water treatment—fundamentals, processes and modeling. DE Gruyter, Berlin
Worch E, Grischek T, Börnick H, Eppinger P (2002) Laboratory tests for simulating attenuation processes of aromatic amines in riverbank filtration. J Hydrol 266(3–4):259–268
Funding
The research was financed by the Ministry of Education, Science and Technological Development of the Republic of Serbia within the project OI 172028.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Jan Schwarzbauer
Electronic supplementary material
ESM 1
(DOCX 32.2 kb)
Rights and permissions
About this article
Cite this article
Maćerak, A.L., Ivančev-Tumbas, I., Börnick, H. et al. Assessment of the retardation of selected herbicides onto Danube sediment based on small column tests. J Soils Sediments 19, 964–972 (2019). https://doi.org/10.1007/s11368-018-2084-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-018-2084-2