Abstract
Purpose
The production of technosols to remediate polluted or sealed urban soils to sustain new green areas is mainly empirical. For this, our research aims to contribute with the scientific knowledge base for purpose designing of technosols. Since iron minerals play an important role for many different functions of soils, we simplified a technique to incorporate and stabilize iron minerals in a substrate: a sand coated with an amorphous iron (hydr)oxide, a 2-line ferrihydrite (2L-FH).
Materials and methods
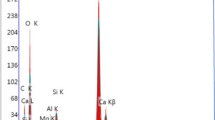
The 2L-FH was precipitated by neutralization of a concentrated FeCl3 solution. The suspension was homogeneously mixed with the sand and the mixture was dried at 35 °C. The mechanical stability of the 21 2L-FH-coated sand was determined by shaking the aggregates in water for 0, 1, 10, 100, and 1 000 min. The degree of coating detachment and the properties of the coating after shaking were characterized through (a) Fe content, (b) zeta-potential and particle size of the detached particles, (c) the specific surface area (SSA) of the coated sand, and (d) its surface structure using scanning electron microscopy (SEM). A phosphate adsorption isotherm was performed to measure the P-sorption capacity of the shaken samples and to test the 2L-FH-quartz attachment stability against the surface charge reduction of the 2L-FH associated with P adsorption.
Results and discussion
A reduced Fe loss (30 %) and smaller sizes of the coating detached particles in the sample shaken for 1 000 min indicate that a fractioning and reattachment of these aggregates occurred during the agitation process, resulting in a smoother surface (SEM), and a larger SSA and P-sorption capacity. The coated shaken samples showed P-adsorption capacities (5.3–6.34 μmol P g−1) comparable to high loadings of phosphate in soils, and low detachment of Fe (7–14 %) in spite of negativity surface charge increase.
Conclusions
The practical novel coating process along with the 1 000-min shaking produced a mechanical resistant and P-adsorptive effective coated sand that could sustain the needs of plants in further experiments.




Similar content being viewed by others
References
Albanese S, Cicchella D (2012) Legacy problems in urban geochemistry. Elements 8:423–228
Arias M, Da Silva-Carballal J, Garcia-Rio L, Mejuto J, Nunez A (2006) Retention of phosphorus by iron and aluminum-oxides-coated quartz particles. J Colloid Interf Sci 295:65–70
Bedarida F, Flamini F, Grubessi O, Pedemonte GM (1976) Hematite to goethite surface weathering scanning electron microscopy. Am Mineral 58:794–795
Bolund P, Hunhammar S (1999) Ecosystem services in urban areas. Ecol Econ 29:293–301
Borggaard OK (1983) The influence of iron oxides on phosphate adsorption by soil. J Soil Sci 34:333–341
Burghardt W (1994) Soils in urban and industrial environments. J Plant Nutr Soil Sci 157:205–214
Brabec E, Schulte S, Richards PL (2002) Impervious surfaces and water quality: a review of current literature and its implications for watershed planning. J Plan Lit 16:499–514
Celi L, Lamacchia S, Marsan FA, Barberis E (1999) Interaction of inositol hexaphosphate on clays: adsorption and charging phenomena. J Soil Sci 164:574–585
Chang YY, Lim JW, Yang JK (2012) Removal of As(V) and cr(VI) in aqueous solution by sand media simultaneously coated with Fe and Mn oxides. J Ind Eng Chem 18:188–192
Cornell RM, Schwertmann U (2003) The iron oxides. Wiley-VCH, Weinheim
Duiker SW, Rhoton FE, Torrent J, Smeck N, Lal R (2003) Iron (Hydr)Oxide crystallinity effects on soil aggregation. Soil Sci Soc Am J 67:606–611
Farrah SR, Preston DR (1985) Concentration of viruses from water by using cellulose filters modified by in situ precipitation of ferric and aluminum hydroxides. Appl Environ Microbiol 50:1502–1504
Fleischer M, Chao GY, Kato A (1975) New mineral names. Am Mineral 60:485–486
Huot H, Simonnot MO, Watteau F, Marion P, Yvon J, De Donato P, Morel JL (2014) Early transformation and transfer processes in a Technosol developing on iron industry deposits. Eur J Soil Sci 65:470–484
Ilg K, Dominik P, Kaupenjohann M, Siemens J (2008) Phosphorus-induced mobilization of colloids: model systems and soils. Eur J Soil Sci 59:233–246
Jerez J, Flury M (2006) Humic acid-, ferrihydrite-, and aluminosilicate-coated sands for column transport experiments. Colloids Surf A Physicochem Eng Asp 273:90–96
Kumar A, Gurian PL, Bucciarelli-Tieger RH, Mitchell-Blackwood J (2008) Iron oxide-coated fibrous sorbents for arsenic removal. J Am Water Works Ass 100:151–164
Lai CH, Lo SL, Chiang HL (2000) Adsorption/desorption properties of copper ions on the surface of iron-coated sand using BET and EDAX analyses. Chemosphere 41:1249–1255
Lo SH, Jeng HT, Lai CH (1997) Characteristics and adsorption properties of iron-coated sand. Water Sci Technol 35:63–70
Lukasik J, Farrah SR, Truesdail S, Shah DO (1996) Adsorption of microorganisms to sand and diatomaceous earth particles coated with metall in hydroxides. KONA 14:87–91
Makhelouf A (2009) The effect of green space on urban climate and pollution. Iran. J Environ Health Sci Eng 6:35–40
McLaughlin JR, Ryden JC, Syers JK (1981) Sorption of inorganic phosphate by iron- and aluminium-containing components. J Soil Sci 32:365–378
Mehra OP, Jackson ML (1960) Iron oxide removal from soils and clays by a dithionite-citrate system buffered with sodium bicarbonate. Clay Clay Miner 7:317–327
Michel FM, Ehm L, Antao SM, Lee PL, Chupas PJ, Liu G, Strongin DR, Schoonen MA, Phillips BL, Parise JB (2007) The structure of ferrihydrite, a nanocrystalline material. Science 316:1726–1729
Nehls T, Rokia S, Mekiffer B, Schwartz C, Wessolek G (2013) Contribution of bricks to urban soil properties. J Soils Sediment 13:575–584
Rokia S, Séré G, Schwartz C, Deeb M, Fournier F, Nehls T, Damas O, Vidal-Beaudet L (2014) Modelling agronomic properties of Technosols constructed with urban wastes. Waste Manage in press
Rommel J (2000) Einflulinearer Alkybenzolsulfonate auf Mobilität und biologische Verfügbarkeit von Cadmium und Kupfer in Boden. Dissertation, Universität Hohenheim
Rusch B, Hanna K, Humbert B (2010) Coating of quartz silica with iron oxides: characterization and surface reactivity of iron coating phases. Colloids Surf A Physicochem Eng Asp 353:172–180
Sakurai K, Teshima A, Kyuma K (1990) Changes in Zero Point of Charge (ZPC), Specific Surface Area (SSA), and Cation Exchange Capacity (CEC) of kaolinite and montmorillonite, and strongly weathered soils caused by Fe and Al coatings. Soil Sci Plant Nutr 36:73–81
Scalenghe R, Marsan FA (2009) The anthropogenic sealing of soils in urban areas. Landscape Urban Plan 90:1–10
Scott TM, Sabo RC, Lukasik J, Boice C, Shaw K, Barroso-Giachetti L, El-Shall H, Farrah SR, Park C, Moudgil B, Koopman B (2002) Performance and cost-effectiveness of ferric and aluminum hydrous metal oxide coating on filter media to enhance virus removal. KONA 20:159–167
Scheffer F, Blume HP, Brümmer GW, Horn R, Schachtschabel P, Welp G, Kandeler E, Thiele-Bruhn S, Kögel-Knabner I, Kretzschmar R (2010) Scheffer/Schachtschabel Lehrbuch der Bodenkunde. Spektrum Akademischer Verlag
Scheidegger A, Borkovec M, Sticher H (1993) Coating of silica sand with goethite: preparation and analytical identification. Geoderma 58:43–65
Schwertmann U (1988) Occurrence and formation of iron oxides in various pedoenvironments. In: Iron in soils and clay minerals. Springer, Netherlands, pp 267–308
Schwertmann U, Taylor RM (1989) Iron oxides. In: Dixon JB, Weed SR (eds) Minerals in soils environments. 2nd ed. Soil Sci. Soc. Am., Madison, pp 379–439
Schwertmann U, Cornell RM (2000) Iron oxides in the laboratory, 2nd ed. Wiley-VCH, Weinheim
Séré G., Schwartz C., Ouvrard S., Sauvage C., Renat JC, Morel JL (2008) Soil construction: a step for ecological reclamation of derelict lands. J Soils Sediment 8:130–136
Strauss R, Brümmer GW, Barrow NJ (1997) Effects of crystallinity of goethite: II. Rates of sorption and desorption of phosphate. Eur J Soil Sci 48:101–114
Sumner ME (2000) Handbook of soil science. CRC Press, Florida
Szecsody JE, Zachara JM, Bruckhart PL (1994) Adsorption-dissolution reactions affecting the distribution and stability of CoIIEDTA in iron oxide-coated sand. Environ Sci Technol 28:1706–1716
Thirunavukkarasu OS, Viraraghavan T, Subramanian KS (2003) Arsenic removal from drinking water using iron oxide-coated sand. Water Air Soil Poll 142:95–111
Trivedi P, Axe L (2001) Ni and zn sorption to amorphous versus crystalline iron oxides: macroscopic studies. J Colloid and Interf Sci 244:221–229
Wang HD, White GN, Dixon JB, Turner FT (1993) Ferrihydrite, lepidocrocite, and goethite in coatings from east texas vertic soils. Soil Sci Soc Am J 57:1381–1386
Wang X, Li W, Harrington R, Liu F, Parise JB, Feng X, Sparks DL (2013) Effect of ferrihydrite crystallite size on phosphate adsorption reactivity. Environ Sci Technol 47:10322–10331
Weng Q, Lu D, Schubring J (2004) Estimation of land surface Temperature-Vegetation abundance relationship for urban heat island studies. Remote Sens Environ 89:467–483
IUSS Working Group WRB (2006) World Reference Base for soil resources 2006, 2nd ed. World Soil Resources Reports No.103, FAO, Rome
White AF (1995) Chemical weathering rates of silicate minerals. Rev Mineral 31:407–462
Xu Y, Axe L (2005) Synthesis and characterization of iron oxide-coated silica and its effect on metal adsorption. J Colloid Interf Sci 282:11–19
Yakovlev AS, Kaniskin MA, Terekhova VA (2013) Ecological evaluation of artificial soils treated with phosphogypsum. Eurasian Soil Sc 46:697–703
Zhao J, Huggins FE, Zhen F, Huffman G (1994) Ferrihydrite: surface structure and its effects on phase transformation. Clay Clay Miner 42:737–746
Acknowledgments
We thank Dr. Sondra Klitzke for her enlightning contribution to the discussion. We thank the Center for Microscopy of the Technische Universität (ZELMI), especially Christoph Fahrenson, for his help on the microscopy images. We thank Claudia Kuntz, Maike Mai, and Monika Rohrbeck for their technical assistance with the laboratory procedures. We thank the Deutsche Akademische Austauschdienst (DAAD, German Academic Exchange Service) and the Ministry of Foreign Affairs of Mexico (SRE Mexico) for funding.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Jean Louis Morel
Rights and permissions
About this article
Cite this article
Flores-Ramírez, E., Dominik, P. & Kaupenjohann, M. Novel ferrihydrite sand coating process as a first step for designed technosols. J Soils Sediments 18, 3349–3359 (2018). https://doi.org/10.1007/s11368-016-1450-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-016-1450-1