Abstract
Purpose
The presence of high copper (Cu) and cadmium (Cd) contamination in soils around mining areas has raised serious health concerns. Improving hydroxyapatite (HAP) adsorption capacity for Cu and Cd is important if its application potential in heavily contaminated soils is to expand.
Materials and methods
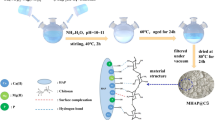
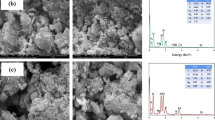
The micro/nanostructured HAP (mnHAP) was synthesized using a template-induced method to improve the HAP immobilization of Cu and Cd in contaminated soils. Commercial and synthetic HAPs were evaluated as amendments in Cu and Cd remediation tests with 1.5 and 3.0 % addition level for 90 days, and soils without HAP materials (0.0 %) were designated as the controls; each treatment was repeated three times. The materials were characterized by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), N2 adsorption, and scanning electron microscopy (SEM)-energy-dispersive spectra (EDS) and then quantitatively determined the Cu and Cd contents by inductively coupled plasma (ICP) and inductively coupled plasma mass spectrometry (ICP-MS).
Results and discussion
The mnHAP was more effective in immobilizing Cu and Cd than the two commercial HAPs. After treatment with mnHAP at the 3.0 % addition level for 90 days, the contaminated soils showed 55.2 and 84.8 % reductions in Cu and Cd concentrations in the toxicity characteristic leaching procedure (TCLP) leaching procedure, respectively. The experimental data indicated that the enhanced Cu and Cd immobilization by mnHAP was due to the increases of surface area and the improvement of structure and newly introduced carboxylate groups on its surface.
Conclusions
These findings show that regulating the structure and surface properties of HAP can enhance Cu and Cd immobilization in soils.







Similar content being viewed by others
References
Amacher MC (2001) Nickel, cadmium, and lead, in: methods of soil analysis: part 3. Chemical methods. In: Sparks DL (ed) SSSA book series 5. Soil Science Society of America, Inc., American Society of Agronomy, Inc, Madison, pp 739–768
Bowman GM, Hutka J (2002) Soil physical measurement and interpretation for land evaluation. Australian Soil and Land Survey Handbooks. CSIRO Publishing, Collingwood, pp 224–239
Cang L, Zhou DM, Wang QY, Wu DY (2009) Effects of electrokinetic treatment of a heavy metal contaminated soil on soil enzyme activities. J Hazard Mater 172:1602–1607
Cao XD, Wahbi A, Ma L, Li B, Yang YL (2009) Immobilization of Zn, Cu, and Pb in contaminated soils using phosphate rock and phosphoric acid. J Hazard Mater 164:555–564
Cheng XK, Huang ZL, Li JQ, Li Y, Chen CL, Chi R, Hu YH (2010) Self-assembled growth and pore size control of the bubble-template porous carbonated hydroxyapatite microsphere. Cryst Growth Des 10:1180–1188
Christoffersen J, Christoffersen MR, Larsen R, Rostrup E, Tingsgaard P, Andersen O, Grandjean P (1988) Interaction of cadmium ions with calcium hydroxyapatite crystals: a possible mechanism contributing to the pathogenesis of cadmium-induced bone diseases. Calcif Tissue Int 42:331–339
Corami A, Mignardi S, Ferrini V (2007) Copper and zinc decontamination from single- and binary-metal solutions using hydroxyapatite. J Hazard Mater 146:164–170
Corami A, Mignardi S, Ferrini V (2008) Cadmium removal from single- and multi-metal (Cd + Pb + Zn + Cu) solutions by sorption on hydroxyapatite. J Colloid Interface Sci 317:402–408
Cui HB, Zhou J, Si YB, Mao JD, Zhao QG, Fang GD, Liang JN (2014) Immobilization of Cu and Cd in a contaminated soil: one- and four-year field effects. J Soil Sediments 14:1397–1406
Da Rocha NCC, De Campos RC, Rossi AM, Moreira EL, Barbosa AD, Moure GT (2002) Cadmium uptake by hydroxyapatite synthesized in different conditions and submitted to thermal treatment. Environ Sci Technol 36:1630–1635
Fedoroff M, Jeanjean J, Rouchaud JC, Mazerolles L, Trocellier P, Maireles-Torres P, Jones DJ (1999) Sorption kinetics and diffusion of cadmium in calcium hydroxyapatites. Solid State Sci 1:71–83
Fernández AA, Pérez CB, Fernández GE, Falqué LE (2000) Comparison between sequential extraction procedures and single extractions for metal partitioning in sewage sludge samples. Analyst 125:1353–1357
Gray CW, Dunham SJ, Dennis PG, Zhao FJ, McGrath SP (2006) Field evaluation of in situ remediation of a heavy metal contaminated soil using lime and red-mud. Environ Pollut 142:530–539
He Q, Huang ZL (2009) Controlled synthesis and morphological evolution of dendritic porous microspheres of calcium phosphates. J Porous Mater 16:683–689
Inoue K, Huang PM (1984) Influence of citric acid on the natural formation of imogolite. Nature 308:58–60
Isoyama M, Wada SI (2007) Remediation of Pb-contaminated soils by washing with hydrochloric acid and subsequent immobilization with calcite and allophanic soil. J Hazard Mater 143:636–642
Joseph S, Graber E, Chia C, Munroe P, Donne S, Thomas T, Nielsen S, Marjo C, Rutlidge H, Pan G (2014) Shifting paradigms: development of high-efficiency biochar fertilizers based on nano-structures and soluble components. Carbon Manag 4:323–343
Lee SH, Lee JS, Choi YJ, Kim JG (2009) In situ stabilization of cadmium-, lead-, and zinc-contaminated soil using various amendments. Chemosphere 77:1069–1075
Leyva AG, Marrero J, Smichowski P, Cicerone D (2001) Sorption of antimony onto hydroxyapatite. Environ Sci Technol 35:3669–3675
Li ZW, Zhou MM, Lin WD (2014) The research of nanoparticle and microparticle hydroxyapatite amendment in multiple heavy metals contaminated soil remediation. J Nanomater 2014:1–8
Li HY, Ye XX, Geng ZG, Zhou HJ, Guo XS, Zhang YX, Zhao HJ, Wang GZ (2015) The influence of biochar species on long-term stabilization for Cd and Cu in contaminated paddy soils. J Hazard Mater. doi:10.1016/j.jhazmat.2015.10.048
Liu GJ, Talley JW, Na CZ, Larson SL, Wolfe LG (2010) Copper doping improves hydroxyapatite sorption for arsenate in simulated groundwaters. Environ Sci Technol 44:1366–1372
Liu G, Deng Q, Wang HM, Kang SH, Yang Y, Ng DHL, Cai WP, Wang GZ (2012) Synthesis and characterization of nanostructured Fe3O4 micron-spheres and their application in removing toxic Cr ions from polluted water. Chem Eur J 18:13418–13426
Lu RK (2000) Soil and agro-chemical analysis methods. Agricultural Science and Technology Press, Beijing, pp 205–266
Lu F, Cai WP, Zhang YG (2008) ZnO hierarchical micro/nanoarchitectures: solvothermal synthesis and structurally enhanced photocatalytic performance. Adv Funct Mater 18:1047–1056
Mandjiny S, Zouboulis AI, Matis KA (1995) Removal of cadmium from dilute solutions by hydroxyapatite. I. Sorption studies. Sep Purif Technol 30:2963–2978
Marchat D, Bernache AD, Champion E (2007) Cadmium fixation by synthetic hydroxyapatite in aqueous solution thermal behavior. J Hazard Mater 139:453–460
Melamed R, Cao XD, Chen M, Ma LQ (2003) Field assessment of lead immobilization in a contaminated soil after phosphate application. Sci Total Environ 305:117–127
Mimmo T, Marzadori C, Montecchio D, Gessa C (2005) Characterisation of Ca- and Al-pectate gels by thermal analysis and FT-IR spectroscopy. Carbohydr Res 340:2510–2519
Mulligan CN, Yong RN, Gibbs BF (2001) Remediation technologies for metal contaminated soils and groundwater: an evaluation. Eng Geol 60:193–207
Nelson DW, Sommers LE (2001) Total carbon, organic carbon, and organic matter. In: Methods of soil analysis. Part 3—chemical methods. Soil Science Society of America, Inc., American Society of Agronomy, Inc., Madison, pp 961–1010
Quevauviller P, Rauret G, Griepink B (1993) Single and sequential extraction in sediments and soils. Int J Environ Anal Chem 51:231–235
Rayanaud S, Champion E, Bernache AD, Thomas P (2002) Calcium phosphate apatites with variable Ca/P atomic ratio I. Synthesis, characterization and thermal stability of powders. Biomaterials 23:1065–1072
Sandrine B, Ange N, Didier BA, Eric C, Patrick S (2007) Removal of aqueous lead ions by hydroxyapatites: equilibria and kinetic processes. J Hazard Mater A139:443–446
Sheng GD, Yang ST, Sheng J, Hu J, Tan XL, Wang XK (2011) Macroscopic and microscopic investigation of Ni(II) sequestration on diatomite by batch, XPS, and EXAFS techniques. Environ Sci Technol 45:7718–7726
Silvano M, Alessia C, Vincenzo F (2012) Evaluation of the effectiveness of phosphate treatment for the remediation of mine waste soils contaminated with Cd, Cu, Pb, and Zn. Chemosphere 86:354–360
Smičiklas ID, Milonjić SK, Pfendt P, Raičević S (2000) The point of zero charge and sorption of cadmium (II) ions on synthetic hydroxyapatite. Sep Purif Technol 18:185–194
Soejoko DS, Tjia MO (2002) Infrared spectroscopy and X-ray diffraction study on the morphological variations of carbonate and phosphate compounds in giant prawn (Macrobrachium rosenbergii) skeletons during its moulting period. J Mater Sci 38:2087–2093
Uchimiya M, Chang S, Klasson KT (2011) Screening biochars for heavy metal retention in soil: role of oxygen functional groups. J Hazard Mater 190:432–441
Usman A, Kuzyakov Y, Stahr K (2005) Effect of clay minerals on immobilization of heavy metals and microbial activity in a sewage sludge-contaminated soil. J Soil Sediments 5:245–252
Wang YJ, Chen JH, Cui YX, Wang SQ, Zhou DM (2009a) Effects of low-molecular-weight organic acids on Cu(II) adsorption onto hydroxyapatite nanoparticles. J Hazard Mater 162:1135–1140
Wang YQ, Wang GZ, Wang HQ, Cai WP, Liang CH, Zhang LD (2009b) Template-induced synthesis of hierarchical SiO2@γ-AlOOH spheres and their application in Cr(VI) removal. Nanotechnology 20:155–604
Xu Y, Schwartz FW, Traina SJ (1994) Sorption of Zn2+ and Cd2+ on hydroxyapatite surfaces. Environ Sci Technol 28:1472–1480
Ye XX, Kang SH, Wang HM, Li HY, Zhang YX, Wang GZ, Zhao HJ (2015) Modified natural diatomite and its enhanced immobilization of lead, copper and cadmium in simulated contaminated soils. J Hazard Mater 289:210–218
Zhang ZZ, Li MY, Chen W, Zhu SZ, Liu NN, Zhu LY (2010) Immobilization of lead and cadmium from aqueous solution and contaminated sediment using nano-hydroxyapatite. Environ Pollut 158:514–519
Zheng W, Li XM, Yang Q, Zeng GM, Shen XX, Zhang Y, Liu JJ (2007) Adsorption of Cd(II) and Cu(II) from aqueous solution by carbonate hydroxylapatite derived from eggshell waste. J Hazard Mater 147:534–539
Zhou DM, Wang YJ, Wang HW, Wang SQ, Cheng JM (2010) Surface-modified nanoscale carbon black used as sorbents for Cu(II) and Cd(II). J Hazard Mater 174:34–39
Acknowledgments
This research was supported by grants from the National Major Research Program of China (Grant no. 2013CB934302), the National Natural Science Foundation of China (No. 41301347 and No. 21177132), the Anhui Provincial Natural Science Foundation (No. 1408085MKL61), the Scientific and Technical Key Research Program of Auhui, Hefei Science Center CAS (2015HSC-UE004), and the CAS/SAFEA International Partnership Program for Creative Research Teams of the Chinese Academy of Sciences, China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Huijun Zhao
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 1141 kb)
Rights and permissions
About this article
Cite this article
Dong, A., Ye, X., Li, H. et al. Micro/nanostructured hydroxyapatite structurally enhances the immobilization for Cu and Cd in contaminated soil. J Soils Sediments 16, 2030–2040 (2016). https://doi.org/10.1007/s11368-016-1396-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-016-1396-3